A joint research group led by graduate student Taishi Oka, Associate Professor Takeshi Maeda, Assistant Professor Naoya Suzuki and Professor Shigeyuki Yagi of the Graduate School of Engineering at Osaka Metropolitan University, Professor Hideki Fujiwara and Assistant Professor Daisuke Sakamaki of the Graduate School of Science at Osaka Metropolitan University, senior researcher Kenji Kamada and Tatsuki Konishi (graduate student at Kwansei Gakuin University at the time of the research) of the Nanomaterials Research Institute of the Department of Materials and Chemistry at AIST, has discovered that a near‐infrared absorbing dye, which had previously been considered a closed‐shell molecule, has an electronic structure between closed and open‐shell. They also revealed that the wavelength of near‐infrared light that can be absorbed becomes longer as the proportion of open‐shell structures increases within the dye.
Provided by Takeshi Maeda, Osaka Metropolitan University
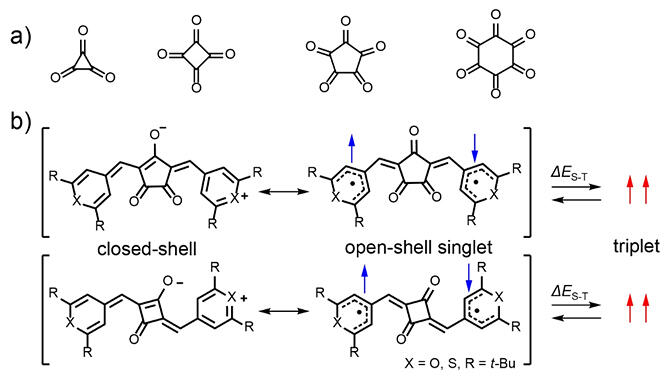
Dyes absorbing near‐infrared light at the boundary between visible and infrared light tend to become more chemically unstable as the absorption wavelength increases, making it a challenge to determine the cause and ensure their stability. The research group employed the recently actively studied method of evaluating open‐shell singlet molecules in order to identify the electronic structure of near‐infrared absorbing organic dyes with a four‐ and five‐member oxocarbon framework at the center of the molecule and a heterocyclic skeleton at the ends of the molecule. They found that proton nuclear magnetic resonance and electron spin resonance spectroscopy measurements showed a remarkable temperature‐dependent change in the resonance signal, indicating that the dye can be excited from singlet to triplet with thermal energy. X‐ray crystallography revealed that the oxocarbon and heterocyclic skeletons contribute to the formation of the open‐shell singlet state.
In the open‐shell singlet state, the two unpaired electrons (diradicals) are bound antiparallel to each other in the singlet state but are excited by heat to a triplet with aligned spin orientation, resulting in a magnetic moment.
Magnetic measurements using superconducting quantum interferometry showed an increase in the magnetic susceptibility with increasing temperature.
Both of these phenomena are characteristic of open‐shell singlet molecules, and they found for the first time that the dye used is in an intermediate state between an open‐shell diradical structure with two unpaired electrons and a closed‐shell structure without unpaired electrons (open‐shell singlet state). Furthermore, they found that the contribution of the open‐shell diradical structure increases as the absorption wavelength of the dye becomes longer.
"In the dyes used in this study, we found a correlation between absorption wavelength and diradicality," said Maeda. "We would like to understand the scope of application and limitations of this correlation and use it in the design of dyes. Also, as the dyes used in this research have both near‐infrared absorption capacity and paramagnetic properties, we hope to develop them into things like photofunctional materials, non‐linear optical materials, semiconductor materials and magnetic materials."
■ Closed‐shell structure: An electron configuration in which two electrons strongly interact with each other and are assigned as a pair to a single molecular orbital. It is an electron configuration possessed by common organic materials.
■ Open‐shell structure: An electron arrangement in which electrons are not assigned to molecular orbitals in pairs. It has unpaired electrons.
■ Open‐shell singlet molecule: A molecule in which the two electrons are not fully paired, but are loosely bound to each other and have a partially paired electron configuration.
■ Singlet and triplet: The spin of an electron has two states: upward and downward. When filling a molecular orbital with two electrons, the triplet is the state in which the two paired electrons have spin in the same direction. Conversely, a singlet is a state in which the two paired electrons have opposite (anti‐parallel) spins.
Journal Information
Publication: Chemical Science
Title: Unveiling a new aspect of oxocarbons: open‐shell character of 4‐ and 5‐membered oxocarbon derivatives showing near‐infrared absorption
DOI: 10.1039/D2SC06612B
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




