A joint research group led by Visiting Scientist Nobuhiro Morishima and Chief Scientist Yoshihiro Ito of the Nano Medical Engineering Laboratory at the RIKEN Cluster for Pioneering Research has developed a method for the sensitive detection of small damage and weak stress in cells and their effects on cell growth and ageing.
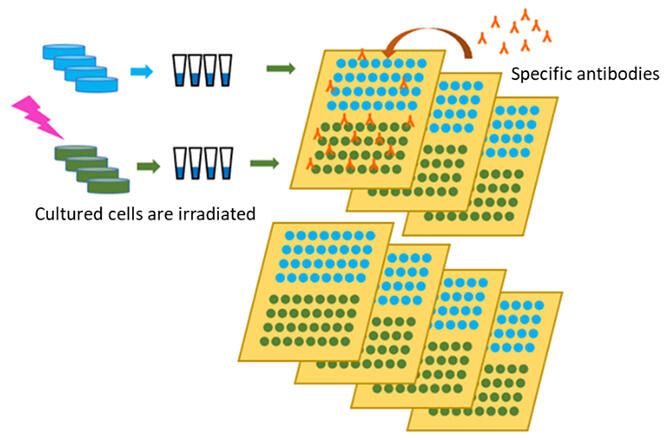
The research group aimed to precisely quantify proteins in irradiated cells by repeatedly measuring a single sample using the advantages of the protein array method, which can handle a large number of samples simultaneously. To apply this method, it would be efficient to use an automated array preparation device (arrayer) to prepare a group of samples (arrays) in which small amounts of cell extract samples are arranged on a substrate, but the availability of arrays for research use is limited. Moreover, the number of spots on a single array ranges from a few hundred to a thousand. In response to this, the researchers developed a new arrayer to create arrays on palm‐sized substrates with the help of Precision Shibasaki.
Provided by RIKEN
The developed arrayer has a stamping head (192 pins) with a size equivalent to half of a 384‐well sample plate, and its movement can be automatically controlled in three dimensions. The cell extract in the sample plate is applied to the pin tip of the stamp head and applied onto a 7 × 8.5 cm nitrocellulose membrane that serves as the substrate. Arrays of up to approximately 7,000 spots can be made repeating such operations as shifting the position of the spot where the cell extractant is applied a little at a time.
The researchers used this arrayer to study the effects of low‐dose‐rate radiation on cells using the low‐dose/low‐dose‐rate irradiation equipment at Kyoto University's Radiation Biology Center. They irradiated human‐derived fibroblasts with gamma rays for 50 hours and compared the number of intracellular proteins, an indicator of the stress response, between irradiated and non‐irradiated cells. The dose rate of the irradiated gamma radiation was 1 milligray per minute. To refine protein quantification, they prepared four cell samples of the same protein in each irradiation experiment using four culture petri dishes (multiple measurements) and each cell sample was spotted eight times on the substrate (repeat measurements). This allows 32 spots to be produced per experimental condition. They repeated the same irradiation experiment two more times on different days (three times in total) to produce arrays consisting of a total of 96 spots per experimental condition. Similarly, they made protein arrays for as many protein types as there were proteins to be examined.
Specific antibodies are added to the protein arrays obtained from this and bind to specific proteins present in the spots. The bound antibody was made to emit light using a fluorescent reagent for antibody detection, and the amount of bound antibody, that is, the amount of the protein to be analyzed, was measured from the intensity of the fluorescence. By arranging all cell samples on the same substrate, proteins could be quantified under uniform conditions.
"We want to try to see if we can detect the effects of even lower levels of radiation on living organisms than those used in this study," explained Morishima. "We hope this method can also be used to analyze small cellular damage and chronic stresses caused by things such as drugs and the environment, provided that the proteins to be analyzed are appropriately selected."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




