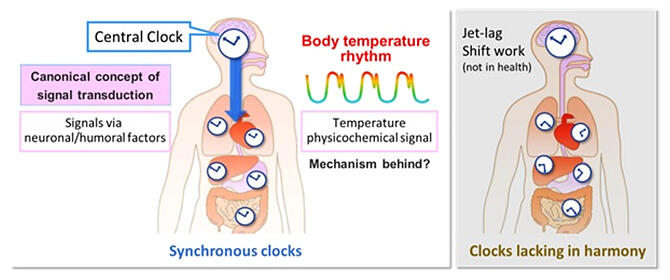
Wake up in the morning, work during the day, sleep at night. Keeping this lifestyle rhythm allows people to maintain their day‐to‐day health. Even at the cellular level, it is important for an exact rhythm to be harmoniously ingrained — if it breaks down, it leads to various illnesses. To enable this, the body's clock receives instructions from a central clock in the brain. Past research has shown that changes in the basal body temperature of a few ℃ inside the body work to bring cells into harmony throughout the body. However, the molecular mechanism behind this was shrouded in mystery. A research group consisting of Assistant Professor Takahito Miyake, Yuichi Inoue (a doctoral student at the time of the research) and Professor Masao Doi of the Graduate School of Pharmaceutical Sciences, Kyoto University has discovered a ribonucleic acid (RNA) sequence needed for the flexible temporal adaptation of the body's rhythm. Their outcomes were published online in Cell Reports.
Provided by Kyoto University
The research group carried out Ribo‐seq analysis using a next‐generation sequencer to investigate the effects of a tiny change in physiological temperature, from 35 ℃ to 38.5 ℃, on the circadian clock gene Per2. Consequently, they found an area with an accumulation of ribosomes for protein synthesis in the 5' untranslated region (UTR), upstream of the region that encodes the PER2 protein in the Per2 messenger RNA (mRNA). Doi explained, 'It was a coincidental discovery. When we altered the temperature of Per2, we found that protein expression was upregulated even though mRNA was not. Miyake investigated this and learned that ribosomes were accumulating in a region in which they do not normally do so.'
When the group looked at this area at single base level resolution, they confirmed that this is a minimal open reading frame that encodes a single amino acid (methionine). This minimal upstream open reading frame (m‐uORF) is generally highly conserved in Per2 in mammals, including humans.
More detailed investigation showed that warm temperature shift (WTS) did not change Per2's mRNA expression or PER2 protein longevity (decomposition speed), but did increase the PER2 protein synthesis rate; in the cells of mice with a Per2 m‐uORF genomic mutation, the increased PER2 expression due to WTS disappeared.
To clarify which molecule mediates the WTS temperature response, the group screened over 150 compounds using a library of kinase inhibitors and identified PI3K as the master regulator. The accumulation of ribosomes in the m‐uORF induced by WTS is controlled by the PI3K inhibitor.
In fact, Per2's m‐uORF sequence and PI3K are needed for phase adaptation due to temperature changes in cells. When the group prepared multiple cultured cells in different phases and subjected these to a changing body temperature cycle actually measured in mice, they assumed a common phase after five days, but it became clear that phase adaptation was inhibited in the Per2 m‐uORF mutant cells and in cells treated with the PI3K inhibitor.
Using a wound‐healing model, the group discovered that the Per2 m‐uORF mutant mouse demonstrated poor healing of a cut to the skin, lacking the time dependency of wound healing seen in regular mice. In other words, this suggests that the PI3K‐Per2 m‐uORF pathway discovered through this study is a key to normal tissue homeostasis inside the body.
Discord in the whole body's internal rhythms is the cause of many illnesses. It is thought that the discovery of a new RNA element that mediates the circadian clock response to physiological temperature will become a foundation for the development of therapeutic drugs based on the harmony of the cellular rhythm across the whole body.
It was unclear what organism‐level physiological functional changes are caused by changes in the translational speed from mRNA. It could be said that this research is the first to clarify the role of RNA sequences that govern the regulation of translational speed in the body at animal level. This could be an important first step in understanding the principle of the flexibility of life through the regulation of translational speed.
Journal Information
Publication: Cell Reports
Title: Minimal upstream open reading frame of Per2 mediates phase fitness of the circadian clock to day/night physiological body temperature rhythm
DOI: 10.1016/j.celrep.2023.112157
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




