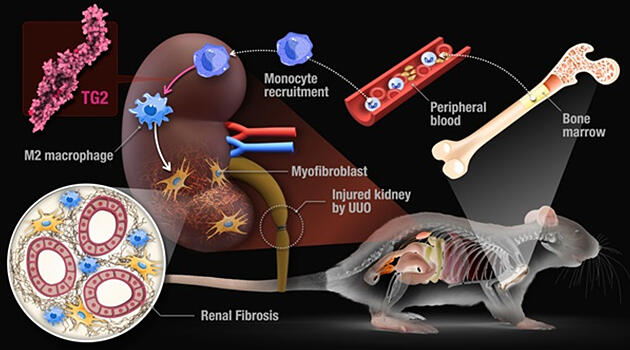
A Nagoya University research group led by Assistant Professor Hideki Tatsukawa, graduate student Yoshiki Shinoda, and Professor Hitomi Kiyotaka of the Graduate School of Pharmaceutical Sciences, Nagoya University, announced that they have identified that 'M2' macrophages, which suppress inflammation, are induced by the protein cross‐linking enzyme transglutaminase TG2 (tissue transglutaminase), causing renal fibrosis that leads to renal failure. Transplantation of bone marrow from TG2‐deficient mice into wild‐type mice with destroyed bone marrow cells inhibited renal fibrosis even when it was induced. The researchers found 'TG2' induces polarization to specific monocyte‐derived M2 macrophages and induces renal fibrosis through the induction of extracellular matrix‐producing myofibroblasts. The findings are expected to lead to the development of therapies for renal failure. The group's research was published in the international academic journal 'Cell Death & Disease.'
Provided by Nagoya University
Macrophages are a type of white blood cell that maintain biogenic homeostasis by cooperating with other immune cells such as through antigen presentation and regulation of inflammatory responses. There are two types: those indigenous to organs, and those derived from monocytes in the bone marrow. The endogenous macrophages at the site of damage function first, but macrophages are also produced by monocytes and sent to the damaged site if the damage is significant. They also undergo a transformation (polarization) with different pro‐inflammatory (M1) and anti‐inflammatory (M2) properties in response to microenvironmental changes, both of which are of various types. These discriminations are made using markers such as surface proteins.
M2 macrophages suppress excessive inflammatory responses and, in the case of chronic inflammation, stimulate the production of collagen and other substances in the repair response, which leads to fibrogenesis. As a disease progresses, the tissue responsible for the filtration function is inhibited by collagen and other factors. As such, in renal disorders, removal of macrophages has attracted attention as a therapeutic target as it improves pathology.
However, fibrogenesis itself is a process of tissue repair, and only drugs that delay the deterioration of the condition have been developed. There is also insufficient knowledge on macrophage polarization, and its molecular mechanisms have been difficult to study because there are few factors in common with those of mice.
The research group therefore focused on TG2, which had been reported to be commonly involved in fibrogenesis in humans and mice. TG2 is an enzyme that forms cross‐linked structures between proteins, forming isopeptide cross‐links with glutamine and lysine residues and is expressed throughout the body.
When the researchers generated TG2‐deficient mice and induced kidney fibrosis, the absolute number of macrophages in the kidneys decreased. In contrast, it increased in renal fibrosis in wild mice. When they analyzed the flow cytometry of the macrophages reduced by TG2 deficiency, they found that TG2 induced M2 macrophages in which specific markers were suppressed.
Furthermore, renal fibrosis was suppressed in TG2‐deficient mice, and myofibroblasts that produce collagen, which promote fibrogenesis, decreased, and collagen itself also decreased.
To determine the origin (indigenous /bone marrow monocytes) and function of these macrophages, the researchers conducted a bone marrow transplant and macrophage transplant into the kidneys.
The results showed more than 95% of the M2 macrophages that increase with renal fibrosis were found to be derived from monocytes. In renal fibrosis, monocytes in the bone marrow circulate in the blood, infiltrate into the injured site of the kidney, differentiate into macrophages, and then are polarized to M2 by TG2.
Furthermore, using a human monocyte cell line, the researchers suppressed TG2 with an inhibitor when induced to M2 type with interleukin‐4 (IL4) after differentiation into macrophages, they obtained macrophages with specific markers suppressed similarly to mice.
To identify the role of TG2 in this polarization, the researchers studied genes whose expression is reduced by TG2 inhibitors and increased in the absence of inhibitors in a comprehensive gene expression analysis. They found several relevant genes, among which the enzyme gene Arachidonate 15‐Lipoxygenase (ALOX15) was found to be significantly over‐expressed. ALOX15 oxidizes arachidonic acid to 15‐hydroxyeicosatetraenoic acid (15‐HETE).
These results indicate that bone marrow monocytes collect in the kidneys and ALOX15 acts at the downstream of TG2 to polarize M2 macrophages and activate fibroblasts, leading to progression of renal fibrosis. Fibrogenesis is also involved in non‐renal dysfunction, and similar molecular mechanisms may be responsible for the disease.
"Now that we have common findings in humans and mice regarding macrophages expressing TG2 and ALOX15, we want to actually look at macrophages in patients with kidney disease in the future," said Tatsukawa. "We want to lead our research to the development of a truly anti‐fibrotic drug with fewer side effects."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




