If we lick salt, it tastes salty, but if we make a very dilute salt solution, it tastes sweet. It may be possible to explain the reasons behind this strange phenomenon. A research group consisting of Nanako Atsumi, Yuriko Takashina, Chiaki Ito, Associate Professor Norihisa Yasui and Professor Atsuko Yamashita of the Graduate School of Medicine, Dentistry and Pharmaceutical Sciences/School of Pharmaceutical Sciences, Okayama University, Professor Keiko Yasumatsu of Tokyo Dental College, Junior College and their colleagues has discovered that the chloride ions that are one of the components of table salt interact with sweet/umami taste receptors to induce taste. Their outcomes were published in eLife.
Provided by Okayama University
We perceive a variety of components within our food through the sense of taste, and recognize five basic tastes: sweetness, umami, saltiness, bitterness and sourness. Within the mouth, we have taste receptors for each of the five basic tastes. For example, the sweet receptors sense and induce the perception of sugar, and the salt receptors do the same for sodium ions, one of the components of table salt. Each receptor has a 'keyhole'‐like pocket in which the substance that induces each taste fits perfectly, and it is thought that the receptors specifically recognize each taste substance when the sugar or sodium ions bond with them, like a 'key.' The salt receptors have no pockets that can bond with sugar, so we do not perceive sugar as being salty. This taste sensory system commonly exists in all vertebrates, from humans to fish.
The taste induced by table salt is known to have strange properties. For example, we perceive the 0.8% to 1% strength salt solution in miso soup as a delicious salty taste, but if this salt solution is diluted tenfold or twentyfold, it tastes sweet—this phenomenon was reported in a psychology research paper around 60 years ago. However, the reason for this phenomenon was completely unknown.
The best way to investigate the relationship between the keys and keyholes of the taste receptors is structural analysis of the shape of the receptor protein at the atomic level. In 2017, the research group from Okayama University clarified the structure of the taste substance sensor domain of taste receptor T1r2a‐T1r3 of the medaka fish (rice fish), first as a taste receptor and then as the same type of receptor as human receptors for sweet and umami tastes. This is currently the only example of structural understanding we have for the sweet and umami taste receptors.
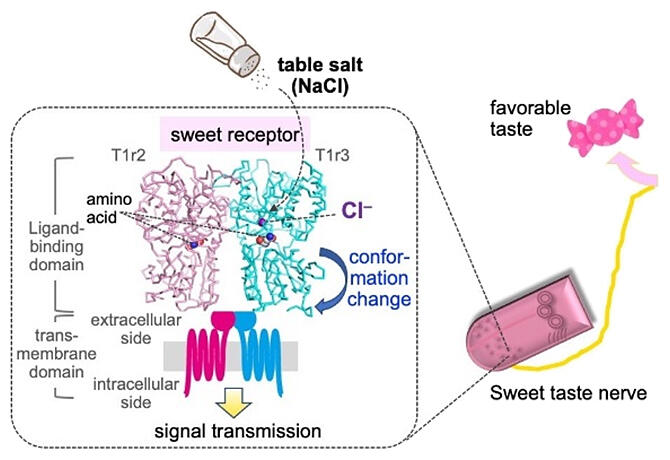
When they investigated this structure in detail, next to the pocket to which amino acids (the taste substance sensed by the medaka fish's receptors) bond, they discovered the existence of a pocket to which a different substance bonds. From the results of analysis using the synchrotron radiation facility SPring‐8 and the Photon Factory, the group found that it is chloride ions that bind to this pocket. This chloride ion bonding pocket is found in T1r3, the structural component common to both the sweet taste receptors and umami taste receptors, and exists in the receptors of almost all animals, including the sweet taste receptors and the umami taste receptors of humans, as well as medaka fish.
It is thought that when a taste substance such as amino acid binds to the sensor domain in sweet/umami taste receptors, the structure of the domain changes, this structural change becomes a trigger, and information about the taste substance is transmitted within the body. The group thus investigated the effect of chloride ions using the receptor proteins of the medaka fish and found that chloride ion bonding induces the same structural changes in the receptor's sensor domain as taste substances such as amino acid. Moreover, Yasumatsu analyzed whether this information is actually perceived as taste within the body using mice taste nerves and learned that chloride ions induce a sweet‐taste nerve response through the mouse sweet taste receptors, meaning it is perceived as a taste.
The concentration of chloride ions needed to induce these effects in the receptors and gustatory nerves is low—a fraction of the concentration that causes the salt taste receptors to sense table salt—but is around the same as the concentration of dilute salt solution that people perceive as having a sweet taste. In fact, the researchers found that mice preferred to drink water containing dilute chloride ions rather than water that contained nothing, and that they perceived the chloride ions as having a pleasant taste similar to sweetness.
Compared with the taste induced by sucrose and similar substances, the taste induced by the chloride ions through the sweet taste receptors is weak. If the concentration of table salt is increased, this causes a phenomenon known as taste mixture suppression, in which the salty taste perceived by the salt taste receptors is stronger and the weaker taste is masked, making us unlikely to notice the sweetness of table salt on a day‐to‐day basis.
Yamashita commented, "When we analyzed the receptors in our mouths that work as sensors to perceive sweet and umami tastes, we found that 'something' was adhering near the taste substance that we fundamentally sense, which triggered our research. Ms. Atsumi, an experimenter with brilliant skills who joined us in our third year and is always smiling, discovered that its true identity is chloride ions, and proved the actual bonding. After that, Ms. Takashina, a student with outstanding research sense who joined us in our seventh year, found that these chloride ions demonstrated the same effect on sweet and umami taste receptors as conventional food substances, which led to this discovery."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




