A research group made up of Professor Norifumi Shioda, Assistant Professor Yasushi Yabuki, Researcher Sefan Asamitsu (currently a RIKEN researcher), and their colleagues from the Department of Genomic Neurology of the Institute of Molecular Embryology and Genetics at Kumamoto University announced that the 'guanine quadruplex (G4)', an RNA structure, forms nucleus of cellular stress granules and that G4 regulates stress granule formation together with G4‐binding protein. The researchers analyzed G4 in the mouse brain and identified a G4‐binding protein, DNA polymerase‐transactivated protein 6 (DNAPTP6). They also found that an artificial suppressor of the protein expression inhibits stress granule formation to induce neuronal cell death. This finding may enable the development of therapies for neurodegenerative diseases. The results were published in 24 Feb 2023 edition of the international academic journal 'Science Advances'.
Provided by Kumamoto University
RNA is involved in various physiological functions, such as translation, mRNA degradation and inflammatory response. A quadruple ‐stranded structure named G4, which contains consecutive guanines, is believed to be present at approximately 3,800 sites among the 2,300 types of mRNA in human cells. However, the function of G4 was unknown. G4 mutations have been reported to cause neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS), but their roles in the central nervous system are also unclear.
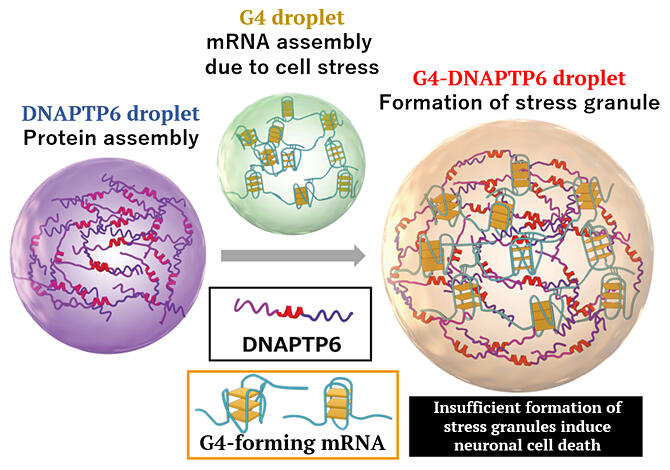
The research group focuses on structures other than the double‐helix formed by genomic DNA and RNA, particularly the G4 structure, and aims to determine the biological role of G4. The researchers extracted and explored the binding proteins that regulate the function of G4 from the mouse brain and found a novel G4‐binding protein, DNAPTP6. Antibody staining of the binding proteins in the mouse brain revealed that the protein was localized in the RNA granules of hippocampal neurons.
The research group then clarified the constituent elements of G4‐forming mRNA using bioinformatics analysis and confirmed that G4 and DNAPTP6 chemically bind to each other. The in vitro intracellular properties of G4‐forming mRNA, DNAPTP6 and G4 were further analyzed. DNAPTP6 and G4‐forming mRNAs were found to aggregate through liquid‐liquid phase separation (LLPS), leading to the formation of stress granules. Stress granules are generated when protein synthesis (translation) by intracellular mRNA is halted because of stimuli, such as heat, oxidative stress and viral infection, and are believed to serve as temporary storage sites for mRNA during translation arrest. This formation adjustment factor had been unknown, but DNAPTP6 was clearly demonstrated to regulate this formation. The results confirmed that artificially suppressing the expression of DNAPTP6 in cultured mouse neurons inhibits the formation of stress granules to induce neuronal cell death.
Shioda stated, "I would like to elucidate hereafter the mechanism by which G4 accumulates in stress granules. I expect that clarifying this mechanism will lead to elucidation of the pathology of neurodegenerative diseases. There are multiple structures other than double helices in nucleic acids, and many of them are still unknown in relation to life phenomena, so I would like to analyze them from various perspectives."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




