A joint research group comprising Assistant Professor Nahoko Kuroki and Professor Hirotoshi Mori of the Department of Applied Chemistry, Faculty of Science and Engineering, Chuo University, Associate Professor Daisuke Kodama of the Department of Chemical Biology and Applied Chemistry, College of Engineering, Nihon University, and Associate Professor Hidetaka Yamada of the Frontier Science and Social Co‐creation Initiative (FSSI), Kanazawa University, along with the Research Institute of Innovative Technology for the Earth (RITE) has succeeded in the theoretical design and rapid materialization of carbon dioxide (CO2)‐absorbing liquids (ionic liquids) with record high physical absorption. This result has been published in The Journal of Physical Chemistry B.
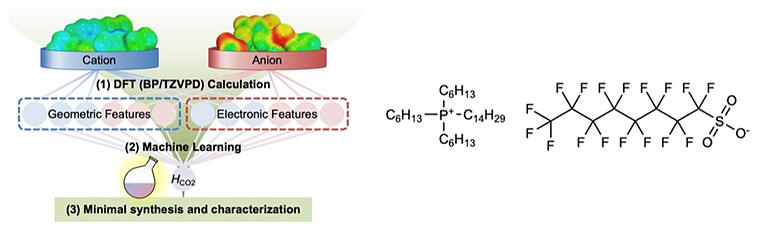
(Right) Molecular structure of ILs exhibiting the record high CO2 physical absorption realized in this study.
Provided by Chuo University
Ionic liquids are room‐temperature molten salts comprising a combination of molecular ions with positive or negative electric charges. Theoretically, more than 1018 different combinations are available, and this number is considerably higher if variations in concentration distributions are considered. Therefore, considering factors such as time and research funds, optimizing the functions of ionic liquids in conventional experiment‐driven research is impractical.
In this research, Mori and his research group applied electronic state informatics, a combination of quantum chemical calculations and machine learning that can precisely simulate intermolecular interactions working in solution/aggregate systems, to rapidly search for ionic liquids with high CO2 solubility from 402,114 ionic liquid candidates.
The feature variables used for machine learning were determined based on the easy‐to‐implement 'quantum chemical calculations of small molecular ion groups,' and the importance of molecular geometries and electronic states governing molecular interactions was quantified based on the wrapper method (machine learning). From this, an explicit consideration of the electronic state of an ion species was clearly demonstrated to be essentially important for the functional design of ionic liquids.
Taking into account the ease with which the synthesis of prospective ionic liquids was narrowed down using machine learning, Yamada's research group then synthesized trihexyl (tetradecyl) phosphonium perfluorooctane sulfonic acid.
A precise and rapid measurement experiment using a magnetic suspension balance owned by Kodama demonstrated that the newly created ionic liquid exhibited higher CO2 solubility than trihexyl(tetradecyl)phosphonium bis(trifluoromethanesulfonyl)amide, conventionally known to have the greatest maximum CO2 absorption.
Therefore, in the future, new ionic species with functional group introductions, elemental substitutions and other modifications can also be systematically considered to accelerate research without being hindered by the enormous chemical space of ionic liquids. The method for developing functional liquids proposed in this study can be applied to other gas‐absorbing liquids, such as deep eutectic solvents or general functional materials composed of multicomponent systems with an extension for weak intermolecular interaction.
Mori stated, "In recent years, we have witnessed the spread of new infectious diseases, abnormal weather conditions and other problems. Due to this, more efficient research than what has been undertaken in the past is desirable. The electronic structure informatics we developed will facilitate the examination of specific materials produced by combining various materials, including new combinations of ions, such as CO2‐absorbing ionic liquids. Using this we can accelerate our research and development with minimum precision experiments. In the future, in cooperation with various companies, we will attempt to implement CO2‐absorbing processes to realize a carbon‐neutral society."
Journal Information
Publication: The Journal of Physical Chemistry B
Title: Machine Learning‐Boosted Design of Ionic Liquids for CO2 Absorption and Experimental Verification
DOI: 10.1021/acs.jpcb.2c07305
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




