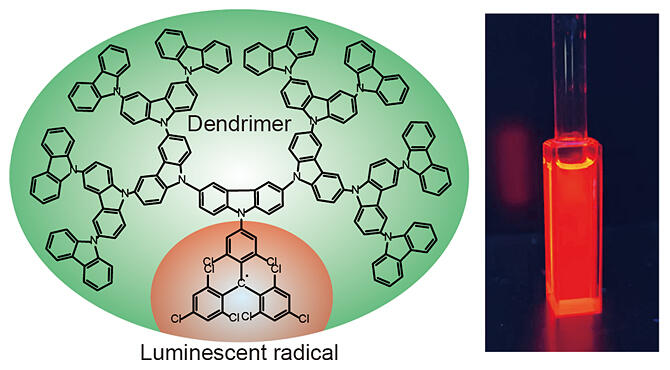
Although most radicals do not emit light, certain radicals do, and these are attracting attention as new light‐emitting materials, mainly in applications to organic EL elements. A research group led by Associate Professor Ken Albrecht of the Institute for Materials Chemistry and Engineering, Kyushu University with Ph.D. student Rui Xiaotian of the Interdisciplinary Graduate School of Engineering Sciences, Researcher Wataru Ota of MOLFEX, Professor Tohru Sato of Fukui Institute for Fundamental Chemistry, Kyoto University, Senior Researcher Takuya Hosokai of the Research Institute for Material and Chemical Measurement at The National Institute of Advanced Industrial Science and Technology (AIST), Associate Professor Yasuo Nakayama of Graduate School of Science and Engineering, Tokyo University of Science and Professor Andrew P. Monkman of Department of Physics at Durham University in the UK succeeded in increasing the luminescence efficiency of the tris(2,4,6‐trichlorophenyl)‐methyl (TTM) radical. The TTM radical is one of the luminescent radicals and its luminescence efficiency was increased from 2 to 63% by binding a dendritic polymer (dendrimer) to it and making it emit red light. The research group also found that binding a larger dendrimer increased the luminance efficiency. A new design method proposed for the generation of highly efficient luminescent radicals was published online in Angewandte Chemie International Edition.
Provided by Kyushu University
Organic molecules that exhibit luminescence are molecules with an even number of electrons, while those with an odd number of electrons are called radicals. The radicals are generally unstable and rarely exhibit luminescent properties. It has been reported that the luminescence and stability of TTM can be improved by binding a TTM radical, which easily accepts electrons, to a donor molecule, which easily gives electrons.
Normal molecules are singlets in the ground state and singlets and triplets in the excited state. Molecules emit either light or heat when they return from the excited to the ground state. Within an organic EL element, 25% of the excited molecules (excitons) produced for emission are singlets, and 75% are triplets. Therefore, to emit light at 100% efficiency, it is necessary to utilize both singlet and triplet excited molecules, which can efficiently emit light back to the ground state. In particular, triplet excited molecules are often non‐luminescent with a tendency to release heat and require a special molecular design to make them luminescent. Conversely, the ground state of a luminescent radical is a doublet, a state with an unpaired electron, and the excited state involved in luminescence is also a doublet. Even within an organic EL element, 100% of the excited molecules produced are doublets, implying that 100% efficiency can be achieved with a simpler mechanism than that for conventional materials. However, luminescent radicals are unstable because they decompose under light irradiation, and there are only a few reports on highly efficient luminescence.
The research group hypothesized that binding a dendrimer with a repeating unit of the carbazole skeleton, known as a donor molecule, to a luminescent radical would improve the luminance efficiency and stability as a result of its asymmetric molecular structure. Experimental results showed that after a decrease in the luminance efficiency with an increase in the size (generation) of a bound dendrimer, the luminance efficiency and stability increased. The emission wavelength also shifted to a shorter wavelength after a longer‐wavelength shift. This was in contrast to the fact that the emission wavelength generally shifts to a longer wavelength on coupling with a large donor with a broadened π‐conjugated system.
Quantum chemical calculations were used to investigate the dendrimer size dependence of the emission wavelength, and a larger dendrimer size was found to facilitate the delocalization of the electrons and decrease the repulsion between them. This effect was caused by the binding of giant dendrimers to radicals and has never previously been reported.
The results of the experiments that investigated the photostability of the luminescent radicals showed that the coupling of dendrimers decreased the degradation rate under light irradiation to less than 1/1000 of the previous value, thus indicating higher stability. In the future, the application of the developed dendrimer‐type luminescent radicals to light‐emitting materials for organic EL devices would contribute to the development of highly efficient devices in the red and near‐infrared regions. This discovery will lead to the creation of more efficient and stable luminescent radicals than ever before and will also contribute to the development of methodologies for designing radicals with the required emission wavelengths.
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




