The ability to use methane, a greenhouse gas abundant in nature, as a hydrocarbon source to replace petroleum will help reduce adverse environmental impacts and overcome resource constraints. In nature, such reactions are performed by enzymes. However, efficiently converting chemically stable methane into useful organic molecules on an industrial scale necessitates the use of a catalyst that simultaneously abstracts proton and electrons. It has been difficult to develop a highly efficient methane oxidation catalyst because the catalyst molecule must be 'electron‐deficient' and possess 'electron‐rich parts.' The properties of such a molecule would sometimes be in a trade‐off relationship.
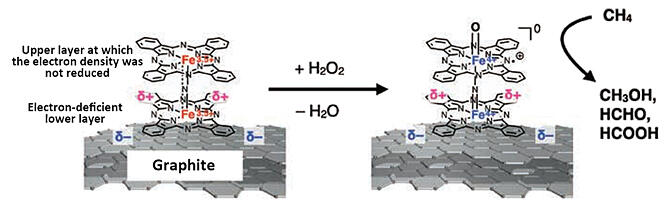
A research group led by Associate Professor Yasuyuki Yamada of the Graduate School of Science at Nagoya University developed a methane oxidation catalyst with a catalytic capability comparable to that of natural methane monooxygenase. More specifically, when a molecule with a cofacially stacked structure was adsorbed onto a graphite surface, the molecule closer to the surface was positively charged as the charge was transferred from that molecule to the graphite, facilitating electron transfer from methane to the graphite. However, because the electron density did not decrease at the most distant molecule (which is responsible for proton abstraction), the catalyst's capacity of proton abstraction was not decreased, whereas its electron abstraction capacity increased.
Although reformation of normal methane requires high temperatures of several hundred degrees Celsius, methanol, formaldehyde, formic acid and other products were successfully synthesized from an aqueous solution of methane, at temperatures below 100℃, using the method of organizing cofacially stacked structure onto the graphite surface. Using this technique, it may be possible to decompose organic materials, such as persistent polymers and waste oil, that cause environmental pollution and unused organic resources, such as lignin, which could be recycled into useful organic materials under mild conditions.
Original article was provided by the Science Portal and has been translated by Science Japan.




