The research group of Researcher Daisuke Kageyama of the Insect Control Technology Group, Division of Insect Advanced Technology, Institute of Agrobiological Sciences, National Agriculture and Food Research Organization (NARO), and Ehime University, in collaboration with Kyoto University, Gunma University, and the National Institute of Health Sciences, announced the discovery of a male‐killing gene in a symbiotic virus of insects. They identified a strain of Drosophila biauraria in which male eggs do not hatch. Analysis of the strain revealed that the non‐hatching was caused by a gene carried by a virus belonging to the family, Partitiviridae, that has a symbiotic association with the insect. Studies of this insect‐virus interaction may lead to the development of pest control technologies. The results have been published in the March 13 issue of the international journal, Nature Communications.
Provided by NARO
Insect cells are known to harbor various microorganisms that are passed on from mother to offspring. For example, Wolbachia bacterium, which live symbiotically in insect cells, manipulates host reproduction by 'male killing' at the embryonic stage or 'feminization' of male embryos.
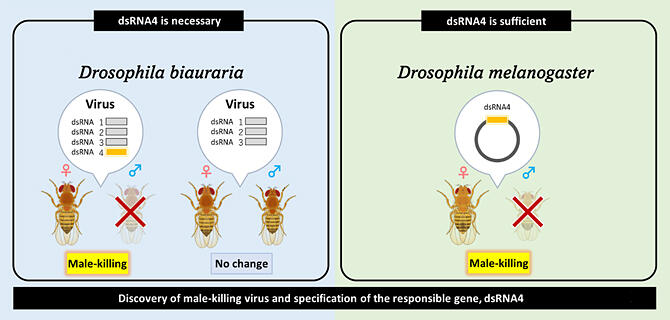
In 2017, NARO and the research group at Ehime University discovered a strain of D. biauraria from the cold regions of Japan, in which only females hatch (males die at the egg stage). The cause of male death was not bacteria, as this strain is inherited by females only, infectious, does not respond to antibiotics, and cannot be detected using polymerase chain reaction. The research group comprehensively analyzed total RNA (RNA‐seq analysis) of the male‐killing strain and normal strains of D. biauraria to determine the cause of male death.
Four RNA fragments with high abundance present only in the male‐killing strain were extracted. The fragments share a common terminal sequence, which is not present in flies. The longest sequence contains information on enzymes required for RNA virus replication. Examination of the intracellular distribution of these four sequences revealed that they co‐localize and are segmented virus sequences that comprise one viral genome. Further database analysis of the viral genome information revealed that the virus is closely related to the family Partitiviridae.
Viruses of the family Partitiviridae contain multiple segments consisting of double‐stranded RNA as the genome and are non‐pathogenic (do not cause disease) in plants and fungi. When a homogenate of the male‐killing strains was injected into the normal strains, strains without female‐killing ability developed over several generations, and the progenies were compared.
The results showed that male killing did not occur when sequence 4 was dropped. Forced expression of the four genes of each sequence in D. melanogaster, a model animal, confirmed the expression of sequence 4 as responsible for male‐killing. The sex ratio of the offspring was normal at 1:1 when the genes of sequences 1‐3 were expressed. This is the first male‐killing gene discovered in a virus, and the genes are not homologous to any male‐killing genes reported to date. Segmented viruses can incorporate genes from other segmented viruses, which may lead to new functions in a virus. The results of this study provide a foundation for evaluating insect sex regulation and developing methods for pest control.
According to Kageyama, "Insect viruses that cause disease have been well‐studied, but the rest are unexplored areas. The Partitiviridae virus family discovered in this study are highly host‐specific and cannot infect D. melanogaster just through injection. My main focus is on Wolbachia and its application, but if we can artificially modify the virus genetically, we can avoid the challenge of host‐specificity; therefore, I would like to advance the development of this technology as well."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/) April 14th Edition. Unauthorized reproduction of the article and photographs is prohibited.




