A research group composed of graduate student Hironobu Sugiyama at the School of Materials and Chemical Technology, Tokyo Institute of Technology; Honorary Professor Hideo Hosono; Professor Masaaki Kitano; Specially Appointed Associate Professor Masato Sasase; and Assistant Professor Masayoshi Miyazaki at the MDX Research Center for Element Strategy has developed a PdMo intermetallic catalyst that promotes carbon dioxide (CO2) hydrogenation reaction at low temperatures and has achieved methanol synthesis under mild conditions of 0.9 MPa and 25 ℃. Their study was published as a preliminary report in the Journal of the American Chemical Society.
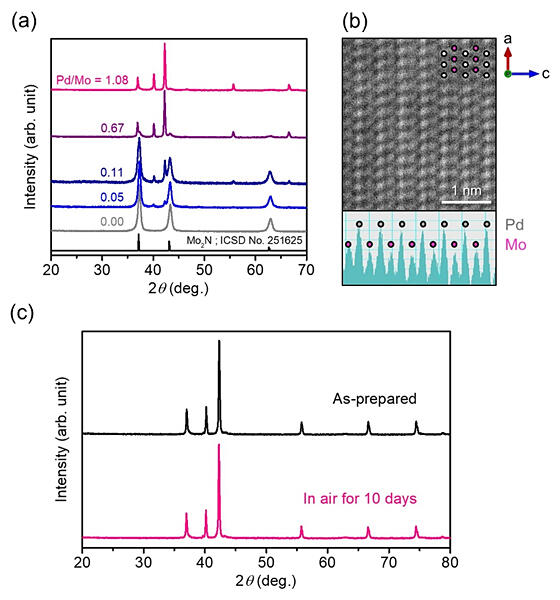
(b) STEM Z‐contrast image obtained in HAADF mode.
(c) XRD patterns for h‐PdMo catalyst before and after exposure to air.
Provided by Tokyo Institute of Technology
Methanol is used as a raw material for fine chemicals and as a fuel additive; it is also expected to become an energy carrier. In recent years, the catalytic process of converting CO2 to methanol has attracted attention from a carbon‐neutral perspective. Catalysts for this catalytic process should meet requirements such as having a simple synthesis method, high chemical stability, accelerated activation of CO2 at low temperatures and easy separation of products. However, an ideal material that satisfies all these requirements has not been reported to date.
The h‐PdMo catalyst, an intermetallic compound with a hexagonal close‐packed (hcp) structure developed by the research group, was easily synthesized via a simple ammonolysis process of oxide precursor and showed excellent durability under atmospheric conditions and a reaction atmosphere. In addition, methanol synthesis was significantly enhanced at low temperatures (below 100 ℃) at which Pd catalysts are not active.
The research group found that an intermetallic compound created by combining palladium (Pd) and molybdenum (Mo) promotes the activation of CO2 under mild conditions and acts as an excellent low‐temperature methanol synthesis catalyst. In this case, intermetallic compounds with the hcp structure could be synthesized by a simple calcination reaction using Pd and Mo oxide precursors in an ammonia atmosphere.
The resulting h‐PdMo/Mo2N, which exhibits high phase stability under atmospheric conditions, was obtained only when the Pd/Mo ratio in the precursor was smaller than 1.08. The methanol synthesis reaction proceeded with the hcp structure, although the synthesis with hydrogen and CO2 did not occur with the Pd/Mo2N catalyst without the hcp structure at normal pressure below 100 ℃. Increasing the pressure improved the synthesis reaction, and continuous methanol production for more than 50 hours even at room temperature (25 ℃) was observed for 0.9 MPa. On the basis of the reaction mechanism analysis, it is suggested that CO2 is firstly decomposed into carbon monoxide (CO) on the catalyst, and then methanol is formed by hydrogenation of this CO.
These research results will have a significant impact on the field, since few reports exist on catalysts capable of synthesizing methanol from CO2 at room temperature. If the CO2 activation mechanism and other details are clarified, further improvements in terms of methanol synthesis activation as well as further acceleration of research on methanol synthesis at low temperatures are expected. The research group stated that the efficiency of the proposed approach remains low and that it is not currently practical; however, they believe that they now see the potential of this technology. They hope to improve the efficiency by employing new ideas in the future.
Journal Information
Publication: Journal of the American Chemical Society
Title: Room‐Temperature CO2 Hydrogenation to Methanol over Air‐Stable hcp‐PdMo Intermetallic Catalyst
DOI: 10.1021/jacs.2c13801
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




