In the chemical industry, hydrogen peroxide is mass‐produced in factories, but when it is synthesized from hydrogen and oxygen on a small scale in laboratories, there are no available catalysts that satisfy the following three conditions simultaneously: (1) the reaction must proceed even when the two gases are mixed in a ratio that does not pose an explosion risk, (2) no complex equipment is required, and (3) the reaction efficiency is high even when the gases are mixed directly.
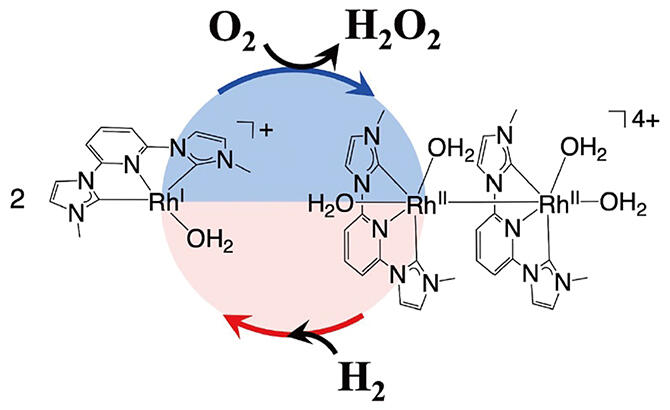
In response to this need, a joint research team led by Distinguished Professor Seiji Ogo of the International Institute for Carbon‐Neutral Energy Research at Kyushu University and Mitsubishi Gas Chemical developed a new molecular catalyst based on a rhodium complex. Hydrogen peroxide is synthesized in nature under mild conditions, as seem in the hydrogenase enzyme that synthesizes and decomposes hydrogen. Inspired by this, a ligand with both electron‐withdrawing and electron‐donating properties was used as a catalyst. This allows the following series of reactions to proceed smoothly: extraction of electrons from hydrogen, oxygen reduction by these electrons, and finally proton extraction from water to produce hydrogen peroxide.
Using this catalyst, hydrogen peroxide can be efficiently synthesized by an aqueous reaction in a single flask without using complicated equipment at a hydrogen/oxygen mixing ratio of 95:5, with almost no risk of explosion. In addition, it was calculated that 910 hydrogen peroxide molecules could be synthesized using a single catalyst molecule, which is the highest reaction efficiency reported to date for homogeneous catalysts. This achievement is not only of academic value in that the group has been able to develop a new molecular catalyst by mimicking the function of a natural enzyme, but also serves as the basis for the development of new synthetic reactions using hydrogen, the next‐generation energy source.
New reaction cycle to produce hydrogen peroxide from hydrogen and oxygen