Cadherin EGF LAG seven‐pass G‐type receptors (CELSR) cadherins, a type of intercellular adhesion molecule, are known to be important in the formation of cell polarity (e.g., the direction in which body hair grows and neurons migrate), cranial nerve networks, and other complex tissues. A research group led by postdoctoral researcher Shigetaka Nishiguchi of the National Institutes of Natural Sciences, Exploratory Research Center on Life and Living Systems (ExCELLS) (currently Specially Appointed Assistant Professor at the Graduate School of Engineering, Osaka University) and Professor Takayuki Uchihashi of the Graduate School of Science, Nagoya University, in collaboration with Specially Appointed Associate Professor Rinshi Kasai of the Institute for Glyco‐core Research, Gifu University, successfully visualized the binding structure of CELSR cadherins at the nanometer scale for the first time in the world. Clarification of the binding mechanism of CELSR cadherins is expected to lead to the elucidation of the principles of complex body formation and the development of diseases caused by impaired CELSR cadherin binding. The findings were published in the Proceedings of the National Academy of Sciences.
Provided by the National Institutes of Natural Sciences
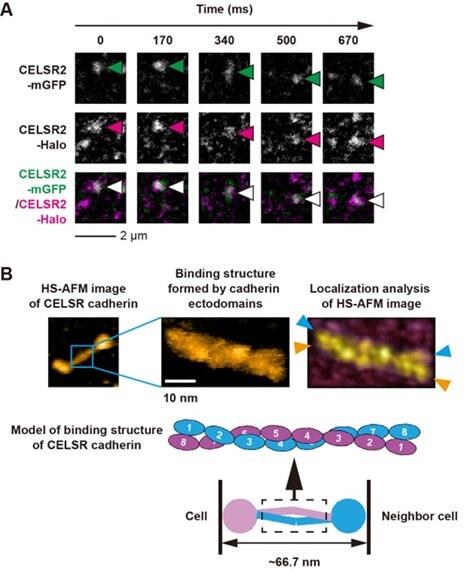
First, the research group obtained two different types of cells, each of which expressed CELSR cadherins labeled with distinct fluorescent molecules. Then, they mixed these two types of cadherins and used single‐molecule fluorescence microscopy to track the movement of these specifically colored CELSR cadherins at the cell‐cell boundary.
The observation revealed that fluorescent molecules from both cell types moved for a long time (∼1 second) and over a long distance (∼1 micrometer) to the same spot at the cell‐cell boundary. This suggests that the two types of cells connect via the binding of their CELSR cadherin molecules with each other.
The structure of CELSR cadherins purified from mammalian cells was then observed using a high‐speed atomic force microscopy (high‐speed AFM), which revealed that the molecules have two characteristic structures: a rod‐like structure consisting of a group of so‐called cadherin domains, and a spherical structure consisting of other domain groups. Furthermore, the bond between two CELSR cadherin molecules was found to be formed via an antiparallel overlap of the cadherin domains of the rod‐like structure.
The antiparallel binding structure of two CELSR cadherin molecules, observed using high‐speed AFM, may reflect the binding state between the two molecules in adjacent cells, as observed by single‐molecule fluorescence microscopy.
The CELSR cadherin image data acquired with high‐speed AFM were further analyzed using the localized AFM method, a recently developed high‐resolution image analysis technique. It was found that the eight CELSR cadherin‐constituting cadherin domains on the distal side (i.e., the side farthest away) of the plasma membrane intertwined and interacted with each other in a helical fashion, forming a bimolecular binding structure.
Investigation of the binding activity of CELSR cadherins using beads revealed that of the eight cadherin domains, the fourth one (counting from the distal side of the plasma membrane) was particularly important for binding between the two molecules.
These results significantly contribute to the elucidation of the mechanism of cell‐cell adhesion by CELSR cadherins; the physiological importance of which has been previously reported.
Although CELSR cadherins are members of the cadherin family, they differ from classical cadherins in their bimolecular binding. As reported previously, classical cadherins require only two cadherin domains for bimolecular binding. By contrast, CELSR cadherins require eight cadherin domains, the largest number reported to date for cell‐cell binding.
The dimer formed by the eight cadherin domains is nearly twice as large as the one formed for classical cadherin. This suggests that CELSR cadherins may act as spacers to maintain a larger distance between cells, which is necessary to allow the passage of extracellular vesicles and other molecules necessary for information exchange between them.
The research group plans to use X‐ray crystallography and cryo‐electron microscopy to 1) clarify the effect of each domain of CELSR cadherin on the binding between two molecules and 2) observe cells expressing liposome‐immobilized CELSR cadherin to analyze the binding structure under conditions that more closely resemble physiological conditions. Additionally, the expression effects of CELSR cadherin molecules lacking certain domains will be analyzed in cultured cells and individual animals.
Journal Information
Publication: PNAS
Title: Antiparallel dimer structure of CELSR cadherin in solution revealed by high‐speed atomic force microscopy
DOI: 10.1073/pnas.2302047120
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




