The research group of Associate Professor Taiho Kambe and graduate student Takumi Wagatsuma of the Graduate School of Biostudies at Kyoto University and Associate Professor Masato Kinoshita of the Graduate School of Agriculture at Kyoto University, in collaboration with Okayama University of Science, Nagoya University and the University of Missouri in the U.S.A., announced that not only copper but also zinc is essential for melanin biosynthesis. This was found by analyzing the melanin synthase TYRP1 and zinc transporter complexes found in killifish (genus Oryzias) and cultured cells. How are metals such as zinc and copper (essential trace elements) related to living organisms? Their findings are expected to clarify the relevant mechanism and were published on 18 April 2023 in Communications Biology.
Provided by Kyoto University
Melanin is the main pigment that determines the color of skin, hair and the iris in the eye and also protects the skin from ultraviolet rays. Two types of melanin are biosynthesized in the body: black eumelanin and red‐orange pheomelanin. The content and ratio of these two types determine the color tone of skin and hair. Melanin is biosynthesized from tyrosine in the melanosomes (organelles) of cells called melanocytes, and three enzymes, namely tyrosinase (TYR), tyrosinase‐related protein 1 (TYR1) and tyrosinase‐related protein 2 (TYR2), are involved in the biosynthetic process. Non‐expression of TYR causes albinism, whereas non‐expression of TYR1 leads to a brownish body/hair color.
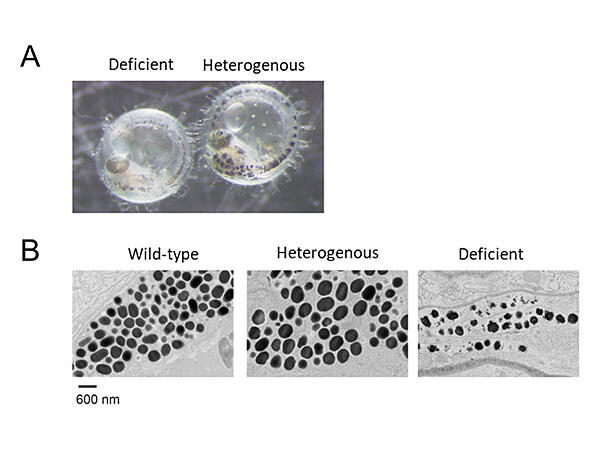
Since around 2002, the research group has been studying the relationship between metal ions that act as essential micronutrients and physiological functions. In 2011, they reported, in collaboration with RIKEN, that the two zinc‐transporter complexes (ZNT5‐ZNT6 heterodimer and ZNT7 homodimer) localized in the endoplasmic reticulum and Golgi apparatus supply zinc to the zinc enzymes. Following this, they have successfully produced a killifish lacking the two zinc‐transporter complexes. The research group noted that this killifish has a lighter color tone than that of the wild type. The melanin amount in the modified killifish was greatly reduced compared with the wild type, and observation by electron microscopy revealed immature melanosomes in the melanocytes of the deficient killifish.
To investigate the involvement of the two ZNT complexes in melanin synthesis, human melanoma cells deficient in both ZNT complexes were prepared. As a result, the defective melanoma strains became lighter and browner in color, and the amount of melanin decreased. In contrast, the normal strain was black. Such brownish alterations have been widely reported in mammals that have mutations in TYRP1. Analysis of the three melanin synthases in human cells lacking both ZNT complexes showed that TYRP1 was not expressed.
When both ZNT complexes were expressed in this deficient strain, the brownish color was restored to black, and TYRP1 expression was also restored. The TYRP1‐deficient strain showed a brown tone, similar to both ZNT complex‐deficient strains, and a similar melanin content. Both ZNT complexes were found to be involved in melanin synthesis through the expression of TYRP1.
Further analysis of the functions of both ZNT complexes was performed. Transient overexpression of TYRP1 in a human melanoma strain lacking both ZNT complexes results in loss of its expression, but simultaneous re‐expression of both ZNT complexes restored TYRP1 expression. The re‐expression of TYRP1 did not occur after the re‐expression of both ZNT complexes that lack zinc‐transporting ability. These findings demonstrate that the zinc transported by both ZNT complexes is essential for the expression of TYRP1. For more than 70 years, it was thought that only copper was important for melanin biosynthesis, but it is now clear that zinc is also essential for normal melanin biosynthesis.
Whitening cosmetics contain a component that inhibits the function of TYR by binding to and chelating copper. It may now be possible to suppress the production of eumelanin, a blackening element, by utilizing a component that chelates zinc.
Kambe stated, "We found that TYR works as a copper enzyme like TYR1. TYR and TYR1 have more than 70% matches in their amino acid sequences, and the two enzymes are almost identical in form, yet they strictly regulate trace amounts of metals. We want to clarify how living organisms recognize trace amounts of metal ions and vice versa. Zinc is not only a structural factor for proteins but is also involved in various functions in the living organisms, and zinc is also reportedly involved in neurodegenerative diseases. I expect that clarifying the mechanism of zinc will lead to a wide range of applications."
Journal Information
Publication: Communications Biology
Title: Pigmentation and TYRP1 expression are mediated by zinc through the early secretory pathway‐resident ZNT proteins
DOI: 10.1038/s42003‐023‐04640‐5
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




