The research team consisting of Independent Scientist Ryota Tamate of the National Institute for Materials Science (NIMS), researcher Yueying Peng of the International Center for Young Scientists (ICYS), NIMS, special researcher Yuji Kamiyama of the Japan Society for the Promotion of Science (JSPS) (third‐year doctoral student of the Graduate School of Life Sciences at Hokkaido University) and Principal Researcher Kei Nishikawa of NIMS has created a polymer gel electrolyte with extremely high mechanical strength and applied it to the protective coating of a lithium metal anode, thereby significantly improving the cycle performance of lithium metal batteries. This will significantly contribute to the practical application of the ultimate anode material: the lithium‐metal negative electrode. Their research results were published in Advanced Materials.
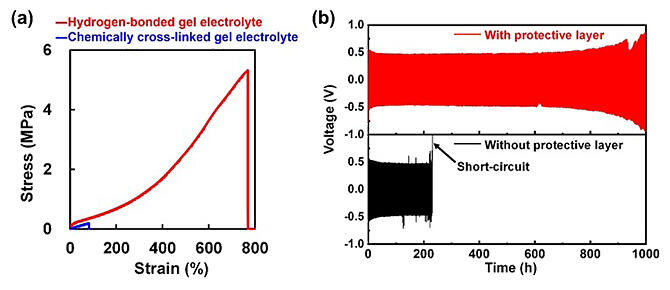
(b) Comparison of cycling behavior between model cells with (red) and without (black) an artificial protective layer made of the gel electrolyte.
Provided by NIMS
While lithium metal anodes have extremely high theoretical capacities and low working potentials, they are prone to incomplete cycles of dissolution and deposition of lithium during charging and discharging, which compromise the charge‐discharge cycle life and safety. Therefore, there is a need for technology to improve the charge‐discharge cycle stability of secondary batteries with lithium metal anodes.
The research team reported a polymer gel electrolyte comprising an organic solvent (organic electrolyte) in which a high concentration of lithium salt is dissolved, along with a hydrogen‐bonding polymer. This polymer gel electrolyte is characterized by extremely high mechanical strength and extensibility. The key to material development is the optimization of the chemical structure and composition of hydrogen‐bonded polymers and the importance of the organic electrolyte composition encapsulated in the gel electrolyte. In particular, the interaction between solvents and polymers has not often been in the spotlight, because most research on polymer gels has focused on hydrogels with water as the solvent.
In this study, the research team found that the polymer structure, as well as the composition of the electrolyte (solvent molecules and ions) that swells the polymer, have a significant effect on the mechanical properties of the gel electrolyte. For example, even when the same hydrogen‐bonded polymer is used, the mechanical strength varies significantly depending on the concentration of lithium salt. This is thought to be because the high concentration of lithium salt increases the proportion of solvent molecules that interact with the lithium ions and decreases the proportion of solvent molecules that interfere with hydrogen bonding between the polymers.
Optimizing the electrolyte and polymer structure and composition based on this concept has resulted in a polymer gel electrolyte with exceptionally high mechanical strength and elongation compared with the polymer gel electrolytes reported thus far. The research team coated the polymer gel electrolyte on the lithium metal negative electrode to form an artificial protective coating and investigated its effect on the performance of lithium metal batteries. Analysis of lithium dissolution and deposition during long‐term cycling of lithium‐symmetric cells showed that the presence or absence of a protective coating has a significant impact on the cycle life. Without the protective coating, the cells short‐circuit after ∼200 hours (h), whereas the introduction of the protective coating allows for longer cycling for more than 1000 h. Electron microscopic observation of the surface of the lithium metal anode with the protective coating after 20 cycles showed that the lithium metal anode was covered with a smooth gel electrolyte coating even after cycling.
Subsequently, a lithium metal battery consisting of an NCM622 cathode (a high‐energy cathode material), and a lithium metal anode was fabricated and compared with and without a protective coating. The introduction of the artificial protective coating significantly improved the cycle characteristics. The use of gel electrolytes with high mechanical strength and extensibility as artificial protective coatings is expected to be an important technology for the use of lithium metal anodes in next‐generation lithium secondary batteries. In addition, the features of this gel electrolyte may also be able to be applied to flexible batteries. However, this research is still in the basic research stage, and there are numerous hurdles and considerations in anticipation of the development of actual batteries. It is also necessary to optimize the thickness of the protective coating and to consider the possibility of applying the coating in other electrolyte systems.
The research team aims to apply the newly developed polymer gel electrolyte to lithium metal batteries as an artificial protective coating and to establish guidelines for optimizing the interface design of batteries with lithium metal anodes.
Journal Information
Publication: Advanced Materials
Title: Extremely Tough, Stretchable Gel Electrolytes with Strong Interpolymer Hydrogen Bonding Prepared Using Concentrated Electrolytes to Stabilize Lithium‐Metal Anodes
DOI: 10.1002/adma.202211679
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




