The group led by Associate Professor Tomoyuki Fukuda, Specially Appointed Lecturer Kentaro Furukawa, Professor Tomotake Kanki of the Graduate School of Medical and Dental Sciences at Niigata University, Researcher Tatsuro Maruyama of the Institute for Microbial Chemistry, and Professor Nobuo Noda of the Institute for Genetic Medicine at Hokkaido University announced the discovery of a novel mitochondrial fission factor, "Mitofissin" (mitochondrial fission protein), in collaboration with the National Institute for Physiological Sciences, RIKEN, and the University of Michigan in the United States.
They established an experimental system in fission yeast and found that Mitofissin cleaves the mitochondrial membrane during mitophagy and splits the membrane into a size that fits into the autophagosome. These advancements are expected to clarify the molecular mechanism of mitophagy and develop novel treatments for mitochondrial diseases. The results were published in the June 15th issue of the international academic journal Molecular Cell.
Provided by Niigata University
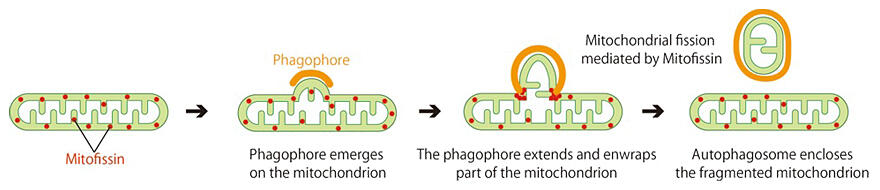
Mitophagy is a phenomenon where autophagy selectively degrades mitochondria, and mitochondria of various sizes within cells are thought to maintain their quality through mitophagy, fission, and fusion.
In 2016, the research group reported that mitophagy does not require a dynamin-like protein that is essential for mitochondrial fission. The group revealed that mitophagy wraps only a part of the mitochondria and forms autophagosomes by a mechanism different from fission.
In the recent study, the researchers used fission yeast instead of budding yeast, which is more common in autophagy and mitophagy research. By establishing an experimental system in fission yeast and utilizing a gene disruption library, they discovered the Atg44 protein, an essential factor for mitophagy, and named it Mitofissin. This factor, a small protein composed of 73 amino acids, is also present in budding yeast. They confirmed that the factor is localized at the mitochondrial intermembrane space. In yeast strains lacking Atg44, mitochondria were found to be swollen and enlarged.
Fluorescence and electron microscopy revealed that, when mitophagy was induced, protrusions similar to those observed during mitophagy accumulated in the deficient strain. In normal mitophagy, the isolating membrane extends to envelop the protrusions and engulf a part of the mitochondria when autophagosomes are formed; however, in the deficient strain, this process halted leaving the extended autophagosome bound with the mitochondria. Furthermore, in the deficient strain, another mitophagy protein marker (Atg32), previously reported by the research group, accumulated around the protrusions. They also confirmed that the factor serves as the mitochondrial fission factor when expressed in human cultured cells.
Based on these results, this factor was suggested to have a function in initiating mitochondrial fission during mitophagy. Due to this, they further analyzed its molecular function. When purified Atg44 was added to artificial lipid nanotubes that imitated the mitochondrial lipid membrane, the lipid nanotubes were cleaved upon binding to the lipid membrane over time. It was found that the factor has the ability to bind and cleave lipid membranes.
To further investigate the cleavage mechanism, they analyzed the crystal structure of the Atg44 monomer and found that one side is hydrophilic, and the other side is hydrophobic. Based on this structure, they simulated how Atg44 binds to lipid membranes and predicted that the monomers, dimers, and tetramers bind to lipid membranes using their hydrophobic face. In addition, when the factor was added to the lipid-film substrate on the basement membrane and was observed using a high-speed atomic force microscopy, they confirmed that the factor accumulated at the membrane periphery, and the membrane moieties were fragmented. It was revealed that the factor directly binds to the mitochondrial lipid membrane and cleaves it by causing fragility.
Kanki stated, "There are still many unrevealed results about mitophagy. We will aim to completely clarify the mechanisms that regulate mitophagy and mitochondrial morphology. Other proteins similar to 'Mitofissin' are likely to exist. If we identify such molecules, we can unveil a novel pathway that controls biological membrane mechanism. In addition, we would like to find a medical treatment to improve the mitochondria function and cure related diseases."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




