A research group led by Assistant Professor Shintaro Mandai, Professor Shinichi Uchida and Takaaki Koide (graduate student) of the Graduate School of Medical and Dental Sciences at Tokyo Medical and Dental University announced that they have found that circulating extracellular vesicles (circulating EVs) with altered properties cause vascular calcification in chronic kidney disease (CKD). The researchers identified a group of microRNAs that have a therapeutic effect by producing animal models of chronic kidney disease and analyzing circulating EVs in the blood. Furthermore, they identified proteins targeted by these microRNAs and confirmed that existing drugs can suppress arteriosclerosis by inhibiting these signals. The results are expected to lead to the development of treatments for cardiovascular diseases associated with CKD. Their research was published in Circulation Research.
Provided by TMDU
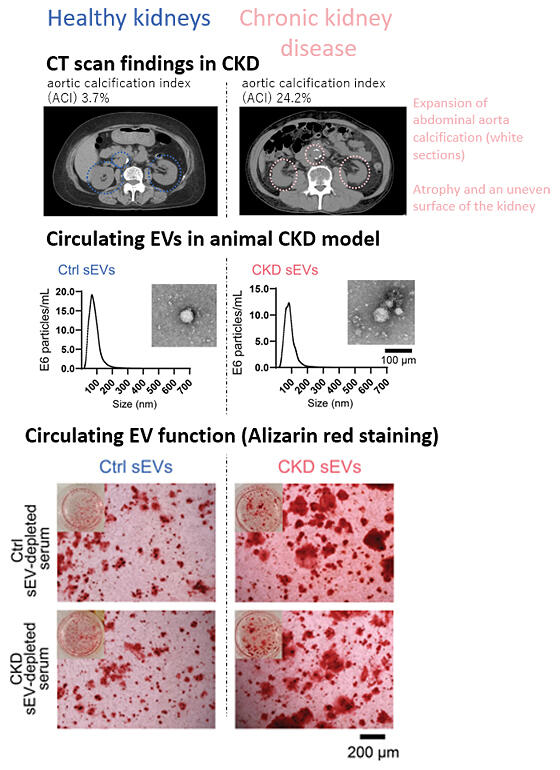
Chronic kidney disease (CKD) affects 13 million people in Japan, one in three elderly people and approximately 700 million people worldwide. It is difficult to become aware of its symptoms and it is estimated that CKD results in more than 340,000 people in Japan being on dialysis. Large Japanese and international studies have shown that arterial calcification progresses as renal function declines, and that the risk of death increases. In fact, based on pathological calcification of arteries, such as heart failure and myocardial infarction, the leading causes of death among dialysis patients is cardiovascular disease. However, the relationship between reduced renal function and pathological calcification has remained a mystery.
The research group has been searching for signaling substances that link the kidneys to other organs in order to understand the pathogenesis of extrarenal organ complications caused by CKD. They have now succeeded in developing a rat model of the disease that develops CKD and confirmed that urinary toxicants such as urea and creatinine are markedly increased in the blood of CKD model rats. They then turned their focus to EVs circulating at high concentrations in the blood and verified the possibility that changes in the quality (inclusions) of EVs occur in CKD and malicious EVs induce vascular calcification.
EVs circulate in high concentrations (more than one billion per cc of blood), encapsulating a variety of functional molecules such as proteins, lipids and RNAs, but their effects on disease are not well understood.
First, the research group compared the particle size and concentration of EVs in the blood of CKD model rats and healthy rats, but found no differences between them. However, when EVs from CKD model rats were added to cultured cells of aortic smooth muscle, the amount of calcium phosphate deposits, an indicator of calcification, increased. In order to investigate the function of circulating EVs in rats, aortic calcification was suppressed by administering a circulating EV production inhibitor to CKD model rats with advanced vascular calcification and confirmed malicious EVs in CKD model rats. The researchers examined the role of microRNAs as a cause of this malicious effect.
A comprehensive analysis of gene expression levels by transcriptome analysis and unique analysis that considers the gene coordinates of microRNAs revealed four types of microRNAs involved in the transformation and calcification of vascular smooth muscle.
In actual CKD patients, these microRNAs were depleted as renal function deteriorated. A sequence information and bioinformatics analysis of the four microRNAs revealed that the common target was VEGFA, a vascular endothelial growth factor.
The researchers also confirmed that VEGFA-VEGFR2 signaling inhibitors suppress calcification and that the existing anti-cancer drug Fruquintinib, which inhibits the same signaling, is effective in suppressing aortic calcification markers in a CKD rat model. The four microRNAs identified in this study all have higher sensitivity and specificity than the existing biomarkers for renal function assessment, such as eGFR and serum phosphorus concentration, and could be used as new biomarkers.
"I believe that we have only just opened the door, but we would like to understand not only microRNAs but also the full range of EV inclusions, so that we can eliminate malicious EVs as a new treatment strategy with fewer side effects, or advance research and development to develop microRNAs and other mimetic drugs with vascular calcification protective effects," commented Mandai.
Journal Information
Publication: Circulation Research
Title: Circulating Extracellular Vesicle-Propagated microRNA Signature as a Vascular Calcification Factor in Chronic Kidney Disease
DOI: 10.1161/CIRCRESAHA.122.321939
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




