Pancreatic cancer is difficult to detect and has a low five-year survival rate, which makes the development of effective therapeutic drugs a high priority. A joint research group led by Associate Professor Suresh Awale of the Institute of Natural Medicine, Professor Naoki Toyooka and Assistant Professor Takuya Okada of the Faculty of Engineering, Professor Tsutomu Fujii and Lecturer Tomoyuki Okumura of the Faculty of Medicine at the University of Toyama, and Lecturer Mitsuro Kanda of the Graduate School of Medicine, Nagoya University has discovered that plumbagin, a component derived from Plumbago auriculata (a member of the Plumbaginaceae family), exhibits selective cytotoxicity against pancreatic cancer cells. Based on this discovery, the group succeeded in creating an innovative pancreatic cancer drug candidate compound with a different mechanism of action from that of existing anticancer drugs. The groups findings were published online in the Journal of Medicinal Chemistry.
Provided by the University of Toyama
Pancreatic cancer has one of the lowest five-year survival rates when compared with other cancer types and is considered the most intractable solid cancer. Because of the deep location of the pancreas and the lack of blood vessels in tumors that form there, pancreatic cancer cells have unique survival mechanisms that allow them to thrive in a cancer microenvironment with limited supply of oxygen and nutrients. This means that existing anticancer drugs such as gemcitabine and paclitaxel, which target rapidly growing cancer cells, are not effective against pancreatic cancer cells. Additionally, serious side effects are a problem with the currently used anticancer drugs, which makes the development of therapeutic agents based on novel mechanisms of action that are effective in the cancer microenvironment is highly desirable.
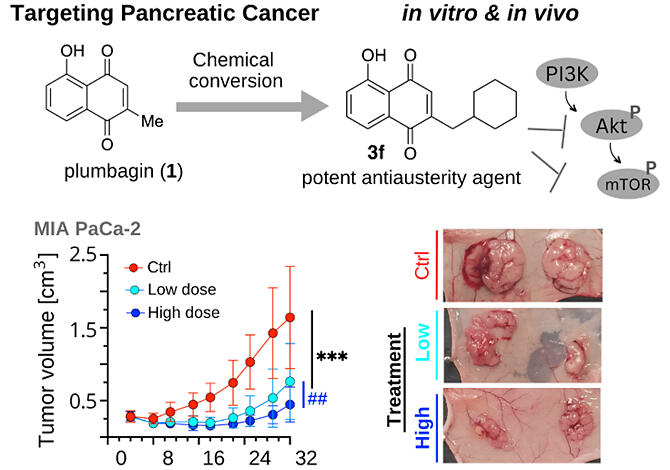
Plumbago auriculata, a species of the dicotyledonous plant family Plumbaginaceae, is widely distributed worldwide, from tropical to arctic regions. The research group observed that plumbagin, a component of Plumbago auriculata, exhibits selective cytotoxicity against pancreatic cancer cells in the cancer microenvironment.

Provided by the University of Toyama
From this background, new compounds based on the chemical structure of plumbagin were synthesized, resulting in the successful synthesis of compound 3f, which exhibits more potent and selective cytotoxicity than plumbagin. Mechanistic studies revealed that compound 3f exerts its cytotoxicity by inhibiting the protein kinase B/mammalian target of rapamycin (Akt/mTOR) signaling pathway. Compound 3f also demonstrated sufficient anticancer activity in animal studies, significantly inhibiting tumor growth in a volume-dependent manner.
These research results demonstrate that the novel plumbagin derivative 3f is highly promising as a new pancreatic cancer treatment drug because of its different mechanism of action from that of the existing anticancer drugs. Further, these findings are substantial to expand the options for pancreatic cancer chemotherapy.
Additionally, the new plumbagin derivative 3f developed by the research group offers new possibilities for multidrug therapy and is expected to help overcome cancer drug resistance, which is a major problem with current treatments. The research group is currently testing the efficacy of compound 3f as a therapeutic agent for pancreatic cancer in clinical trials.
Journal Information
Publication: Journal of Medicinal Chemistry
Title: Targeting Pancreatic Cancer with Novel Plumbagin Derivatives: Design, Synthesis, Molecular Mechanism, In Vitro and In Vivo Evaluation
DOI: 10.1021/acs.jmedchem.3c00394
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




