A research group led by Associate Professor Makoto Ikeya and Program-specific Researcher Denise Zujur of the Department of Clinical Application at the Center for iPS Cell Research and Application (CiRA) at Kyoto University, in collaboration with Professor Koichi Nakayama of the Center for Regenerative Medicine Research at Saga University, Ajinomoto, and other researchers, has developed a method to produce cartilage cell clusters, known as cartilage spheroids, using iPS cell-derived mesenchymal stem cells (iMSC).
By adding low molecular weight compounds, iMSCs were efficiently induced to become cartilage cells (chondrocytes). When the resulting cartilage spheroids were transplanted into mice with an impaired immune system, they were maintained as cartilage tissue, without signs such as dedifferentiation or hypertrophy. The spheroids obtained by this method have the property of fusing together within a few days and can produce large pieces of cartilage tissue. This is expected to be useful for cartilage repair therapy. The results of this study have been published in the 10 May 2023 issue of the international journal Frontiers in Cell and Developmental Biology.
Provided by Kyoto University
Articular cartilage plays a role in the smooth movement of joints, but because it lacks blood vessels, it does not heal spontaneously when it is damaged, leading to joint pain and dysfunction of limbs due to deformity. Replacement with an artificial joint made of metal or plastic is the most common treatment, and this treatment is very effective for pain relief and reacquisition of gait function.
However, due to concerns about durability, transplantation of artificial joints tends to be undertaken cautiously in young individuals and sport enthusiasts. In contrast, regenerative medicine that cells taken from the patient's own body are cultured, and transplanted, however, only partial regeneration of small regions has been achieved, and for application to wide-ranging cartilage deletions and osteoarthritis, both of which are adaptations of joint prosthesis, there are issues related to complications derived from the cells that are raw materials, limited proliferative capacity, or dedifferentiation. Consequently, artificial joint replacement has yet to be in practical application not only in Japan but also in overseas.
For this reason, efforts have been made to develop a technique by which high-quality cartilage cells can be produced from iPS cells.
Last year, the research group established a technique by which iMSCs could be generated from iPS cells, via neural crest cells, under conditions that do not include animal-derived components. Based on their previous results, the group aimed to establish a technique for generating cartilage-like tissue from iMSCs.
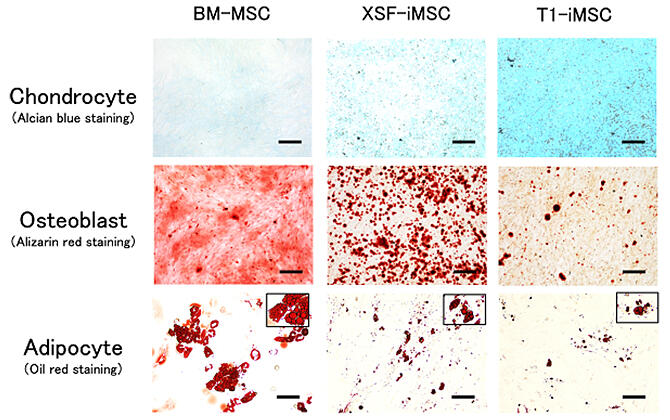
First, to produce iMSCs, the team, in collaboration with Ajinomoto, introduced a new T1 medium that does not contain animal-derived components. Three types of culture medium were used to grow the cells: T1 medium without animal components, commercially available XSF medium, and bone marrow derived MSC, and the rates of induction to become cartilage, bone, and fat cells were compared. The results showed that the iMSC produced using T1 medium had a higher induction efficiency for generating cartilage cells and a lower induction rate for generating bone and fat cells than the other two types.
The team investigated a three-step induction method, in which the culture components were gradually changed to differentiate into cartilage spheroids, to improve the efficiency of induction into cartilage cells further and compared this method with the conventional method. The addition of a low molecular weight compound (TD-198946), which has been reported to promote differentiation of cells into chondrocytes, was also examined. Specifically, cartilage spheroids were generated by comparing a culture method in which TD was added during the last step of a three-step induction method with a conventional method in which the same medium containing TD was used from start to finish.
They found that the three-step induction method led to generation of high-quality cartilage spheroids with greater production of a molecule that serves as a marker of articular cartilage (PRG4) and less production of a molecule that is a marker of overgrown cartilage (COL10A1), which is replaced by bone, indicating that this method was superior to the conventional method.
The cartilage spheroids produced by this three-step method are approximately 0.5 mm in size. In addition, iMSC produced using T1 medium and cartilage spheroids induced by the three-step induction method from bone marrow-derived MSC were transplanted subcutaneously into immunocompromised mice and observed after 8 weeks to see if they remained intact. For the cartilage to function, the cartilage spheroids need to be maintained as they are after transplantation.
They found that the cartilage spheroids generated from iMSC produced using T1 medium were maintained as cartilage spheroids. However, cartilage spheroids induced using bone marrow-derived MSC showed calcification and formation of bone-like tissue in addition to cartilage tissue. In addition, cartilage spheroids produced by the three-step induction method using T1 medium have a property to fuse together if they remained proximal to each other, and they fused together completely after 7 days.
This property is advantageous to produce large pieces of cartilage tissue that is suitable for transplantation. Chondrocytes can form via two pathways during development, involving differentiation from neural crest cells or from mesoderm. The neural crest cells are evolutionarily conserved only in vertebrates.
Ikeya said, "The cartilage spheroids produced in this study have the characteristic of adhering to adjacent cartilage spheroids from day 1, and of adhering to each other by day 4 to the extent that the boundaries are not visible. Taking advantage of this characteristic, we will first attempt to construct centimeter-sized cartilage cell pieces using a bio 3D printer, using the Kenzan method developed by Professor Nakayama, a co-researcher on the project. Since this printer can adjust the shape of the cartilage cells produced, we expect that, if large pieces of cartilage tissue can be constructed, it will be possible to use this approach to treat large cartilage defects."
Journal Information
Publication: Frontiers in Cell and Developmental Biology
Title: Enhanced chondrogenic differentiation of iPS cell-derived mesenchymal stem/stromal cells via neural crest cell induction for hyaline cartilage repair
DOI: 10.3389/fcell.2023.1140717
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




