A research group made up of Associate Professor Kosuke Suzuki, Project Research Associate Kentaro Yonesato, Graduate Student Daiki Yanai and Professor Kazuya Yamaguchi of the School of Engineering, in collaboration with Associate Professor Daisuke Yokogawa of the Graduate School of Arts and Sciences of the University of Tokyo, and Professor Seiji Yamazoe at the Graduate School of Science of Tokyo Metropolitan University and Rigaku, has developed hybrid molecular catalysts combining metal nanoclusters with surface-exposed silver and ring-shaped metal oxides.
The newly developed hybrid molecular catalysts were stable in solid state and solution despite the presence of surface-exposed silver. Moreover, the catalysts can dissociate molecular hydrogen into electrons and protons through the concerted action of silver nanoclusters and surrounding ring-shaped metal oxide. Therefore, they can be used for the conversion of various organic molecules using molecular hydrogen. There are expectations that these findings will lead to applications in material development and were published in Nature Chemistry.
Provided by the University of Tokyo
The properties and functions of metal nanoclusters depend on the number and arrangement of their constituent atoms. Furthermore, metal nanoclusters are expected to have a wide variety of applications that differ from the typical applications of single metal atoms or metals.
In particular, the newly developed metal nanoclusters are critical materials that can work as catalysts by utilizing their high surface reactivity. For example, metal nanoclusters and metal particles can be combined with metal oxides as supports to fabricate catalytic materials. The catalytic materials can be used to synthesize useful chemicals from fossil resources and natural resources or for environmental cleanup. However, surface-exposed metal nanoclusters are often unstable and can easily decompose and aggregate.
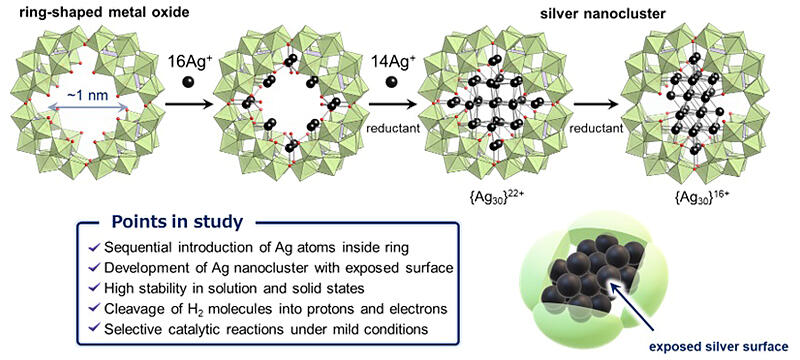
The research group synthesized silver nanoclusters comprising 30 silver atoms within a 1 nm diameter area inside a ring-shaped metal oxide via a stepwise reaction of the ring-shaped metal oxide, silver ions, and a reducing agent.
Furthermore, by reacting silver nanoclusters with a reducing agent, the number of electrons and the arrangement of silver atoms within the silver nanoclusters were changed, thus inducing changes in the structure of the silver nanoclusters.
Single-crystal X-ray structure analysis was used to evaluate the structure of the hybrid molecular catalysts comprising ring-shaped metal oxides and silver nanoclusters. The surface of the silver nanoclusters at the aperture of the ring-shaped structure is not protected by ligands or other similar structures, meaning that an abundance of silver atoms are exposed at the surface. These exposed silver nanocluster surfaces are critical as active sites during catalytic reactions. However, since silver nanoclusters are subject to decomposition and structural changes, it has been challenging to keep them stable with the materials that have been developed so far.
However, UV−visible absorption spectroscopy in organic solvents and X-ray absorption fine structure experiments revealed that the newly-synthesized hybrid molecular catalysts were stable and retained their molecular structure with exposed silver nanocluster surfaces in solid state and solution.
The silver nanoclusters are surrounded by a ring of metal oxide, which is hypothesized to promote the stability of the silver nanocluster surface structure. They exhibited remarkable catalytic properties for the reduction of various organic molecules under moderate reaction conditions, that is lower temperatures and hydrogen partial pressures than those required by conventional silver nanoparticle catalysts.
Unlike common silver nanoparticle catalysts, the hybrid molecular catalyst selectively reduced specific fragments of organic molecules. For example, even in the presence of easily reduced fragments, such as triple carbon bonds or halogens, nitro groups (NO2) were selectively reduced to amino groups (NH2) to form anilines.
The concerted action of silver nanoclusters and ring-shaped metal oxides promoted the dissociation of molecular hydrogen into protons and electrons. Therefore, the catalytic properties of the hybrid molecular catalyst were different from those of common silver nanoparticles.
The newly developed hybrid molecular catalyst exhibits excellent stability and high chemical reactivity. Moreover, the silver nanoclusters and surrounding metal oxide ring work synergistically to confer the material various functions, such as the ability to dissociate molecular hydrogen and catalytic activity. Therefore, in addition to its application as a catalyst for resource recycling, energy conversion, chemical synthesis (e.g., medical and agrochemical products and organic compounds), and environmental cleanup, the hybrid molecular catalyst is expected to be used in numerous fields to fabricate photo-functional materials and sensors, and for molecular electronics applications.
The expectation for the future is that the newly developed synthesis method will enable control over the types of elements and the number of atoms that comprise materials. This could lead to the development of materials with a variety of structures and electronic states customized for specific functions and applications.
Journal Information
Publication: Nature Chemistry
Title: Surface-exposed silver nanoclusters inside molecular metal oxide cavities
DOI: 10.1038/s41557-023-01234-w
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




