With the rise in obesity, there is a global increase in the number of patients with non-alcoholic fatty liver disease (NAFLD) caused by excessive cholesterol accumulation. A proportion of NAFLD patients progress to non-alcoholic steatohepatitis (NASH), which is characterized by inflammation and fibrosis. In Japan, with the westernization of lifestyles, the prevalence of NAFLD diagnosed using physical examination is estimated to be 9-30%, and approximately 10% of these patients have NASH. Liver cirrhosis and liver cancer develop in 10-20% of patients with NASH. However, there is no effective treatment other than diet control and exercise because factors contributing to the development of NASH remain unclear.
Professor Koh Ono and Assistant Professor Takahiro Horie of the Department of Cardiovascular Medicine at the Graduate School of Medicine at Kyoto University have found that microRNA-33b (miR-33b) in hepatocytes plays an important role in the development of NASH. Furthermore, using a mouse model of NASH development, they have shown that administration of a synthetic nucleic acid that specifically inhibits 33b can suppress inflammation, fibrosis, and liver damage, demonstrating its effectiveness in the treatment of NASH. Ono said, "We will continue to develop the drug for therapeutic applications in the future." The study has been published online in Science Alliance.
Provided by Kyoto University
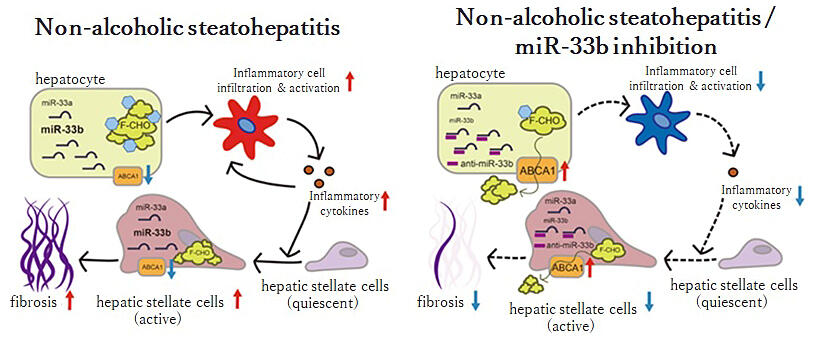
The research group has previously shown that mouse miR-33 suppresses the expression of the intracellular cholesterol transporter ABCA1. ABCA1 transports cholesterol from the intracellular to the extracellular compartment of the cell, forming good cholesterol. Through this, the activation of miR-33 suppresses ABCA1 and lowers good cholesterol levels. In fact, atherosclerosis and aortic aneurysm formation were suppressed in mice lacking miR-33.
However, while there is one type of miR-33 in mice, there are two types in humans, with 33a in the same location as that in mice and 33b in a different location. The role of 33b was a mystery.
The research group generated humanized knock-in mice in which 33b was inserted in the same location as that in humans. Both 33a and 33b were expressed in these mice, and atherosclerosis was worse than that in the wild type. Comprehensive lipid analysis of the mouse livers revealed that lipid levels were higher in the 33a-expressing mice than in the wildtype and lipid levels were even higher in the knock-in mice expressing both 33a and 33b than in the 33a-expressing and wildtype mice.
Knock-in mice were fed a high-fat diet for fat accumulation in the liver, inducing inflammation, fibrosis, and NASH. In addition, there was excessive accumulation of cholesterol and triglycerides in the liver. In contrast, the hepatocyte-specific deletion of 33b improved the NASH phenotype, whereas the macrophage-specific deletion of 33b did not improve the phenotype.
Horie commented, "We have shown that knock-in mice fed with a high-fat diet are an ideal mouse model for NASH."
Next, artificial nucleic acids that could individually inhibit 33a + b in vivo were generated. Further, these artificial nucleic acids inhibiting 33a, 33b, and 33a + b were administered subcutaneously once every two weeks to knock-in mice while they were fed a NASH-inducing diet for 12 weeks.
Effects of administration were confirmed at 21 weeks of age. High levels of liver cholesterol accumulation, inflammatory cell infiltration, and cytokines were observed in the control mice, resulting in the activation and fibrosis of hepatic astrocytes, whereas cholesterol accumulation was suppressed in each mouse subcutaneously injected with the artificial nucleic acid. In particular, inflammatory cytokines and fibrosis genes were more effectively inhibited by the suppression of 33b, which is highly expressed in the liver, than by the suppression of both 33 a + b.
Horie added, "In this experiment, we have found that humanized miR-33b knock-in mice can develop NASH, and miR-33b in hepatocytes is particularly important."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




