Unruptured intracranial aneurysms have been reported in approximately 5% of the Japanese population, however, the etiology and pathology of an aneurysm leading to subarachnoid hemorrhage remain unclear. The role of somatic gene mutations in the development of the following two types of intracranial aneurysms is unknown: Saccular aneurysms, which account for ≥90% of all intracranial aneurysms, have a balloon-like shape, and fusiform aneurysms (<10%) have a rugby ball-like shape.
An international research group led by Senior Scientist Yasuyuki Shima, Team Leader Hirofumi Nakatomi, (Professor at the Faculty of Medicine, Kyorin University) Visiting Scientist Nakao Ota, (Professor at the Graduate School of Medicine at the University of Tokyo) at the RIKEN Center for Brain Science, Research Scientist Shota Sasagawa and Team Leader Hidewaki Nakagawa at the Laboratory for Cancer Genomics at the RIKEN Center for Integrative Medical Sciences; Professor Nobuhito Saito of the Graduate School of Medicine at the University of Tokyo, Project Assistant Professor Yeon-Jeong Kim and Professor Toshihisa Ohtsuka of the Faculty of Medicine at University of Yamanashi, identified somatic gene mutations important for the onset of intracranial aneurysms based on an analysis of human cerebral aneurysm specimens, and established the first mouse models of intracranial aneurysm formation and prevention using gene transfer. Currently, the treatment options for intracranial aneurysms are limited to craniotomies and endovascular catheter treatment. The findings from this study could lead to a drug treatment as a third option. Nakatomi aims to translate the study findings into clinical practice within 10 years and start a clinical trial in five years. These developments were published in Science Translational Medicine.
Provided by RIKEN
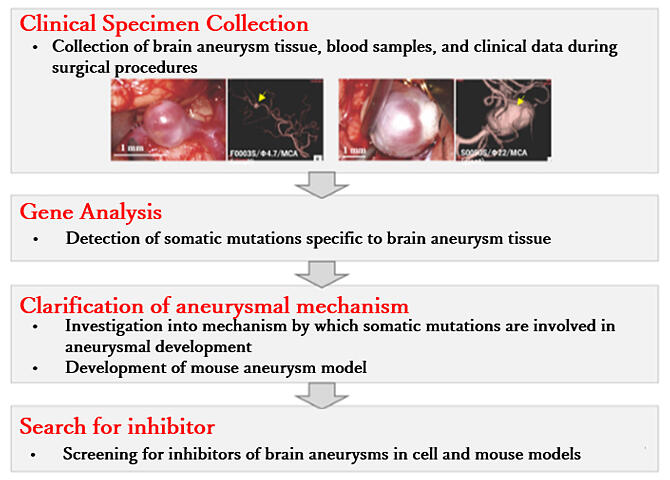
The study group first performed a comprehensive genetic analysis of human cerebral aneurysm surgical specimens obtained from 65 patients and discovered somatic mutations in 405 genes. This study was the first to identify an association between genes other than platelet-derived growth factor receptor beta (PDGFRβ) and intracranial aneurysms. The research group also conducted various genome-wide association studies, including pathway analysis, to examine the associations between the 405 genes and discovered that mutations in 112 of the genes are mutually involved in the onset of intracranial aneurysms. The mutations were found mostly in cancer-related genes. Sixteen mutated genes found in 92% of the analyzed specimens were linked to the nuclear factor kappa B (NF-κB) signaling pathways, which are involved in inflammatory responses and tumor formation. Of the 16 mutated genes, 6 were common in both fusiform and saccular aneurysms. Furthermore, the research group revealed that the PDGFRβ gene is selectively mutated in the most intractable intracranial aneurysms.
Comparisons of clinical data and the gene analysis results of the cerebral aneurysm specimens showed that 4 of the 11 fusiform aneurysm cases with poor prognosis had mutations in the PDGFRβ gene. Moreover, this study demonstrated a new finding regarding saccular aneurysms, in which no genetic mutation had previously been reported. In this study, PDGFRβ gene was mutated in two of the three cases of large/giant saccular aneurysms (≥20 mm, the most intractable type).
In addition, the research group demonstrated a high prevalence of PDGFRβ mutations in pericytes (stem cells on the vascular wall) located on the adventitia of aneurysms. PDGFRβ is a type of receptor tyrosine kinase. Mutations in PDGFRβ lead to upregulation and activation of autophosphorylation, influencing the expression of genes located in the downstream of the signaling pathway. Proliferation and thickening of autophosphorylation-causing pericytes were found in both fusiform and saccular intracranial aneurysm tissues with PDGFRβ mutations. These specimens showed an increased expression of the PDGFRβ gene as well as genes related to inflammation.
The research group then explored potential drugs that inhibit signaling upregulated by the PDGFRβ mutations. The research group treated a PDGFRβ-mutated human cell line with sunitinib (a tyrosine kinase inhibitor to treat kidney cancer) since many inhibitory agents targeting receptor tyrosine kinases, including PDGFRβ, are being developed for cancer treatment. The outcomes demonstrated that sunitinib blocked autophosphorylation.
Lastly, to examine whether PDGFRβ mutations detected in humans induce intracranial aneurysms in mice, a mutated PDGFRβ gene was introduced to the cerebral artery wall in mice via a viral vector. Twenty-eight days after mutated gene induction, a fusiform aneurysm-like change (vascular dilatation) was observed in the basilar artery of the mice. Administration of sunitinib to the mouse model prevented aneurysm formation. This study established the first mouse models of intracranial aneurysm formation and prevention using gene transfer.
Journal Information
Publication: Science Translational Medicine
Title: Increased PDGFRB and NF-κB signaling caused by highly prevalent somatic mutations in intracranial aneurysms
DOI: 10.1126/scitranslmed.abq7721
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




