Anticancer agents induce apoptosis in cancer cells to exert their function. However, therapy-resistant cancers and metastatic cancers become vulnerable to ferroptosis although they develop resistance to anticancer agents targeting existing apoptosis. Therefore, cancer therapies that induce ferroptosis in cancer cells and enhance their sensitivity has attracted worldwide attention. Dr. Eikan Mishima of Tohoku University (Senior Scientist at the Helmholtz Zentrum München), and Graduate Student Toshitaka Nakamura of the Helmholtz Zentrum München, have published two articles regarding this topic in Nature.
Provided by Tohoku University
Dr. Brent Stockwell discovered ferroptosis in 2012, which is a form of cell death in which the cell membrane is destructed owing to excessive lipid oxidation in cells. There are mechanisms in vivo that inhibit ferroptosis, which suppress excessive lipid oxidation: the glutathione peroxidase (GPX4) pathway and the ferroptosis suppressor protein 1 (FSP1) pathway. Ferroptosis can be induced if these pathways are inhibited. However, since GPX4 lacks structurally suitable binding pockets for inhibitors, normal cells may also be affected. FSP1, on the other hand, can develop normally in deficient mice, suggesting that no serious side effects are expected and contains multiple binding pockets for drugs. Dr. Marcus Conrad, head of the lab that Mishima and Nakamura belong to, and his team developed an FSP1 inhibitor, iFSP1, but it was rapidly degraded in vivo resulting in it being ineffective.
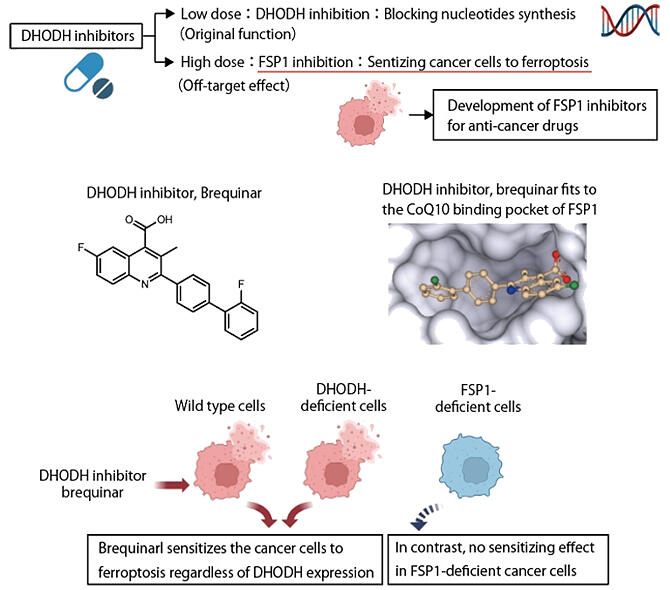
Mishima uncovered the mechanism of action for dihydroorotate dehydrogenase (DHODH) inhibitors, which had been reported to enhance the sensitivity of ferroptosis in cancer cells.
DHODH is an enzyme necessary for synthesizing nucleic acids. It has been reported to suppress ferroptosis by reducing coenzyme Q10 (CoQ10) within the mitochondria. A DHODH inhibitor, brequinar, is undergoing clinical trials as a treatment for cancer to suppress cell proliferation targeting diseases such as leukemia. However, in human cell and mouse experiments, sensitivity to ferroptosis cannot be enhanced unless it is administered at a dosage 600 times higher than the required dose for DHODH inhibition.
Since brequinar and iFSP1 have similar structures, Mishima speculated that "this might be an off-targeting effect," and continued his research.
When FSP1 and drugs were mixed in vitro to evaluate enzyme activity, high concentrations of brequinar also inhibited FSP1. It was also found that brequinar fits into the CoQ10-binding pocket through predictive structural analysis of the CoQ10 binding pocket of FSP1. Conversely, in FSP1 knockout cells, no enhancement of ferroptosis was observed. Furthermore, similar effects were also confirmed in other DHODH inhibitors.
Specifically, it was revealed that DHODH inhibitors serve also as FSP1 inhibitors at high concentrations, and that inhibition of FSP1 enhances the sensitivity of cancer cells to ferroptosis.
Nakamura discovered a novel FSP1 inhibitor, icFSP1, and confirmed that it promotes cell death by ferroptosis.
Using FSP1-expressing GPX4-deficient cells, in which ferroptosis is induced upon inhibition of FSP1, approximately 10,000 compounds were screened to identify icFSP1. It was confirmed that icFSP1 induces ferroptosis in various cancer cells in a concentration-dependent manner. Observation using fluorescence microscopy to examine the mechanism of action revealed that when icFSP1 was added, FSP1 was separated from other proteins and aggregated, triggering phase separation to induce ferroptosis. The observation also confirmed that intraperitoneal administration of icFSP1 to melanoma-engrafted mice attenuated tumor growth.
Nakamura stated, "Since the in vivo stability is insufficient with this drug, we are working on developing compounds with enhanced stability in vivo through different chemical modifications."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




