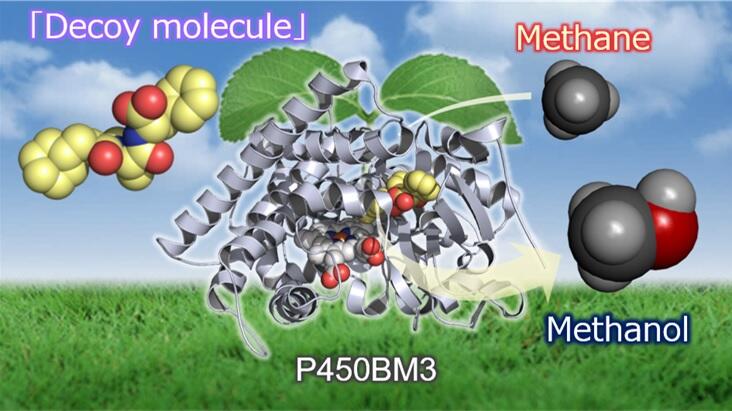
A research group led by Professor Osami Shoji, Assistant Professor Shinya Ariyasu and Graduate Student Kai Yonemura of the Graduate School of Science at Nagoya University announced that they have successfully developed a technology to convert methane, the main component of natural gas, into methanol using an enzyme at room temperature in water. They utilized a unique 'substrate misrecognition system' that artificially controls the function of the enzyme. The research group demonstrated that cytochrome P450BM3, a metalloenzyme derived from Bacillus megaterium that originally hydroxylates long-chain fatty acids, can be converted to methanol by incorporating a 'decoy molecule' resembling a synthesized long-chain fatty acid together with methane. These results are expected to lead to the effective utilization of methane gas, and were published in the June 14, 2023 issue of ACS Catalysis, the American Chemical Society's journal of catalytic chemistry.
Provided by Nagoya University
Methane is the main component of natural gas and is abundant on the earth, but its chemical stability means that at present it requires enormous amount of energy for chemical conversion, and its use has not progressed. Methane hydrates exist in the seas around Japan, and if these can be converted to methanol at low energy, it is expected to lead to an energy revolution. Enzymes that function at room temperature and in water are expected to be used, but the enzyme that converts methane to methanol (methane monooxygenase) is difficult to handle and cannot be mass-produced. It was also believed that enzymes were like a key and a keyhole, with the characteristic of accepting only specific compounds, and that other enzymes could not be used.
In response to this, the research group has designed artificial molecules called 'decoy molecules' that resemble the specific compounds to be chemically converted by enzymes and developed a 'substrate misrecognition system' that tricks enzymes into taking up the decoy molecules and activating them to chemically convert compounds that are not the original targets.
Using the enzyme P450BM3, which hydroxylates long-chain fatty acids derived from Bacillus megaterium abundant in nature, they had succeeded in the chemical conversion of benzene to phenol and the highly efficient hydroxylation of ethane and propane at room temperature and in water. P450BM3 is a metalloenzyme containing iron (Fe) in its reaction center, which is highly reactive and has characteristics that allow for mass production through E. coli expression.
The 'decoy molecule' is designed to be shorter than molecule to be chemically converted, and the compound to be reacted is pressed into the decoy molecule to activate the enzyme as if it were immobilized with a plug. To realize methane hydroxylation with this enzyme, the research group searched for 'decoy molecules' with a structure that can fix methane molecules of the smallest size in a library of about 600 types of 'decoy molecules' that they have designed.
The applicability of about 40 types of molecules, mainly those whose effectiveness has been confirmed by ethane hydroxylation, was verified. The results confirmed that gas chromatography could detect methanol using several 'decoy molecules.' With the most efficient decoy, the research group confirmed that 4 methane molecules can be converted to methanol per enzyme molecule at room temperature and in water. It is thought that there are tens of thousands of P450 enzymes in nature, but no enzyme that could change methane to methanol had been identified.
In this research, methane hydroxylation was achieved for the first time in the world, although the reaction efficiency is still low and there are some separation issues because the methanol produced is in water. Pressure is applied during the current reaction, but methane is small and difficult to confine, so the research group aims to improve the yield by making this improvement in the future. Research on methane hydroxylation is being conducted around the world because of its usefulness, and other research groups have used different approaches, such as genetic modification, but none of them have been successful.
Ariyasu said, "This technique, in which the keyhole is partially filled and the shape of the keyhole can be freely changed, can create various shapes by changing the decoy molecule. We expect that this technology will contribute to the development of environmentally friendly material conversion technologies that will enable the creation of functional molecules, such as functional polymer materials and aroma chemicals, with a low environmental impact."
Journal Information
Publication: ACS Catalysis
Title: Catalytic Oxidation of Methane by Wild-Type Cytochrome P450BM3 with Chemically Evolved Decoy Molecules
DOI: 10.1021/acscatal.3c01158
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




