The research group led by Professor Masahiro Sonoshita of the Division of Biomedical Oncology at the Institute for Genetic Medicine at Hokkaido University has created Drosophila models of pancreatic cancer that mimic the four gene mutations observed in patients with the disease and announced that they have successfully identified a novel therapeutic target after conducting exhaustive screening experiments. They succeeded in reproducing mutations of the four genes in Drosophila (4-hit flies). Using this technique, they performed a comprehensive genetic screen of all kinases to identify new therapeutic targets and confirmed that several developed inhibitors improved survival rates in the 4-hit flies. The results are expected to lead to the development of therapeutic agents for pancreatic cancer. The results were published in the June 28 issue of Cancer Research, a journal of the American Association for Cancer Research (AACR).
Provided by Hokkaido University
Pancreatic cancer is an intractable (i.e., hard to manage) disease with extremely limited treatment options. Activation of the Kirsten rat sarcoma viral oncogene homolog (KRAS) gene and inactivation of the tumor suppressor genes encoding tumor protein 53 (TP53), cyclin dependent kinase inhibitor 2A (CDKN2A), and mothers against decapentaplegic homolog 4 (SMAD4), whether alone or in combination, are observed in this cancer type. Patients with mutations in these four genes have the worst prognosis. As there are no model mice that can reproduce the pathology of pancreatic cancer, creation of other animal models of the disease is needed and expected.
Drosophila possesses more than 70% of the abnormal genes observed in humans. Aside from having a highly conserved genetic structure with mammals, this fruit fly also has a functionally corresponding body structure.
In 2019, the research group used a Drosophila model in which gene mutations for medullary thyroid cancer were introduced to perform genetic and compound screening experiments. Furthermore, the researchers identified kinase inhibitors that could become novel treatment targets and therapeutic drug candidates.
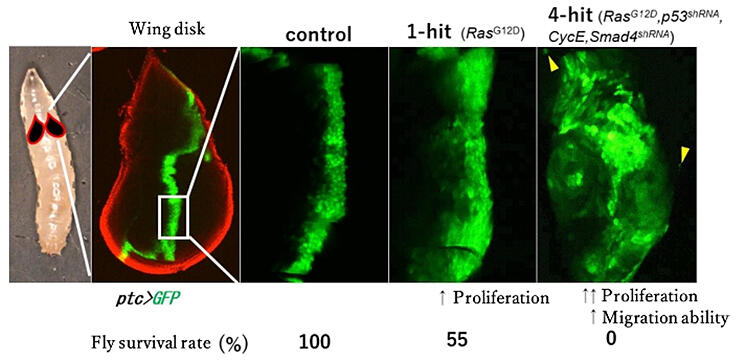
The research group has now succeeded in creating the world's first model fly (4-hit fly) that reproduces mutations of four genes involved in pancreatic cancer. Activation and functional reduction of the fly genes corresponding to each human gene were reproduced in the larval wing primordia, either singly or in combination.
First, they generated 1-hit flies in which only the Drosophila Ras gene (corresponding to KRAS in humans) was activated in the larval wing primordium epithelial cells, and the phenotypes were analyzed. Consequently, cell proliferation accelerated, and approximately half of the flies died. Furthermore, when 4-hit flies were produced, the proliferative and migratory abilities of the wing primordium epithelial cells were remarkably enhanced, and all individuals died without reaching eclosion. From these results, it was confirmed that flies could consistently reflect the pathology of clinical pancreatic cancer.
A comprehensive genetic screen of all kinases was then performed using these 4-hit flies. Kinase abnormalities are found in most types of cancer and are considered to be deeply involved in the abnormal cell proliferation activity of cancers. In their analysis, the research group created 4-hit flies that lacked the function of each kinase gene one by one and analyzed the pathological changes. Humans have approximately 500 kinase genes, whereas flies have approximately half of this number.
The researchers found that reducing the activities of mitogen-activated protein kinase kinease (MEK) and Aurora kinase B (AURKB) improved the survival rate of the 4-hit flies.
Accordingly, the researchers orally administered an MEK inhibitor (trametinib) and/or an AURKB inhibitor (BI-831266) to the 4-hit flies to inhibit the activity of the respective kinases.
As a result, the survival rate of the 4-hit flies was better recovered by the combined administration of the two agents than by the single administration of either inhibitor. Additionally, the research group also confirmed that the co-administration of the two agents markedly suppressed tumor growth, even in model mice implanted with human pancreatic cancer cells. Further analysis of surgical specimens from pancreatic cancer patients at Hokkaido University Hospital showed that the prognosis of patients with a measurable level of phosphorylated histone H3 (an indicator of AURKB activation) had a worse prognosis than those without a detectable level of this protein.
The research group has already identified several other potential novel therapeutic targets.
Sonoshita stated, "We believe that the results have shown the possibility of drug discovery based on the screening of the phenotypic system in Drosophila. Experiments that are difficult to perform in mammals can be efficiently conducted in flies, and we believe that this method is applicable to malignant diseases other than pancreatic cancer. We will develop therapeutic drugs for pancreatic cancer and aim to understand the overall picture of the signaling pathways involved in the disease onset."
Journal Information
Publication: Cancer Research
Title: Drosophila Screening Identifies Dual Inhibition of MEK and AURKB as an Effective Therapy for Pancreatic Ductal Adenocarcinoma
DOI: 10.1158/0008-5472.CAN-22-3762
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




