The key to the practical application of artificial photosynthesis, which uses solar energy to reduce and recycle carbon dioxide (CO2), is to improve the performance of photosensitizers, which absorb light and transfer electrons from an electron source to a catalyst. At present, the photosensitizer role is usually assigned to a single molecule. However, there are limitations in imparting multiple characteristics, such as excellent visible light absorption capability and stability, to single molecules.
Assistant Professor Shin-ya Takizawa, Professor Emeritus Shigeru Murata and Professor Jun Terao of the Graduate School of Arts and Sciences at the University of Tokyo, together with their colleagues have created a new development guideline for photosensitizers that features both excellent visible light absorption capability and high durability, which are essential to advance artificial photosynthesis technology. Specifically, the research group successfully utilized easily controllable iridium (Ir) complexes as photosensitizers and enhanced their performance by pairing two types of Ir complexes, one positively charged and the other negatively charged, thereby complementing each other's functions. This technique is expected to be applicable to not only Ir complexes but also other metal complexes that are abundant on earth and organic compounds that do not contain metals, thereby contributing to the advancement of artificial photosynthesis technology. The results were published in the Journal of the American Chemical Society.
Provided by the University of Tokyo
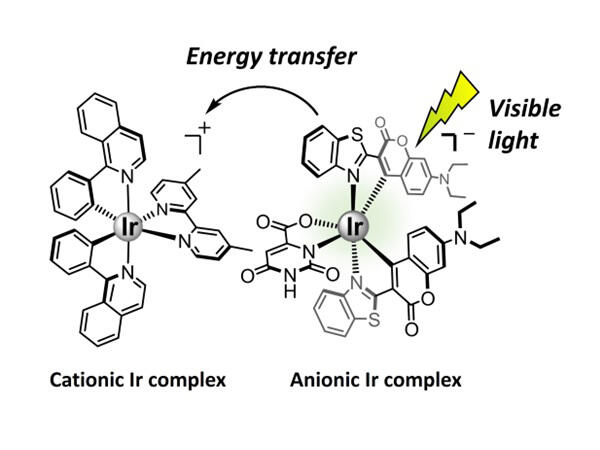
The research group has developed a method utilizing easily controllable Ir complexes as photosensitizers. Here, two types of Ir complexes, one positively charged (Cationic) and the other negatively charged (Anionic), are brought close together by Coulomb force to achieve synergistic functions. First, cationic and anionic Ir complexes with different characteristics were selected, and the corresponding ion pairs were easily synthesized by simply mixing them in methanol. The selected cationic Ir complexes are known to be relatively stable as photosensitizers in the CO2 reduction reaction despite their inability to absorb visible light.
By contrast, the anionic Ir complexes show weaker durability when used alone as photosensitizers, but they have much better visible light absorption capability owing to the inclusion of an organic dye (coumarin 6) in their backbone.
The results of nuclear magnetic resonance spectroscopy revealed that the two Ir complexes approached each other in chloroform and were rearranged. Moreover, owing to their ability to emit light upon light absorption, energy was efficiently transferred from the anionic Ir complex, which absorbs light and enters a high-energy state, to the cationic Ir complex.
This finding indicates that the anionic complex acts as a visible-light-harvesting antenna, assisting the cationic complex, whereas the cationic complex enhances the durability of the anionic complex.
These ion pairs were applied as photosensitizers for the CO2 reduction reaction. Specifically, the ion pairs were incorporated into the lipid-bilayer surface of vesicles along with rhenium catalyst molecules. Ascorbic acid ions were added as an electron source, and the reaction vessel was filled with CO2 and exposed to visible light.
When evaluated in solvents commonly used for CO2 photoreduction reactions, the ion pairs became surrounded with and separated by solvent molecules, thereby hindering the expected effects. Due to this, a lipid bilayer was utilized to overcome the problem. Utilizing the lipid bilayer as the reaction environment enabled the CO2 photoreduction reaction to occur in water.
The results of verification experiments showed that more carbon monoxide was generated as a CO2 reduction product when the cationic and anionic Ir complexes were used together as the photosensitizer than when either complex was reacted separately.
Furthermore, the use of spectroscopic methods to track the state of the reaction solution revealed that the decomposition of the anionic component of the ion pair was significantly suppressed compared with that in reactions using the anionic Ir complex alone. This finding represents a crucial achievement, demonstrating that the ion-pair effect actually contributes to the high performance of photosensitizers.
The new method eliminates the need to synthesize complex compounds from two molecules connected by covalent bonds, which requires significant costs and time. Given suitable molecules that are either commercially available or easily synthesized, the ion pairs can be prepared by simply mixing them together. There is room for the comprehensive exploration of various combinations of molecules with positive or negative charges, and such explorations are expected to provide further support for other investigations of superior photosensitizers in the future.
Journal Information
Publication: Journal of the American Chemical Society
Title: Ion Pairing of Cationic and Anionic Ir(III) Photosensitizers for Photocatalytic CO2 Reduction at Lipid-Membrane Surfaces
DOI: 10.1038/s41598-023-37211-z
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




