A joint research group including Senior Research Scientist Masuki Kawamoto, (Associate Professor of the Graduate School of Science and Engineering at Saitama University), Team Leader Yoshihiro Ito at the RIKEN Center for Emergent Matter Science (CEMS), and their colleagues has discovered a perovskite compound that can be safely used to store ammonia through structural changes. The results have been published in the Journal of the American Chemical Society.
Provided by RIKEN
Ammonia has attracted considerable attention as a hydrogen carrier because of its high hydrogen mass and volume densities. However, ammonia is a highly corrosive gas at room temperature and under ordinary pressure. This means that storage and handling are challenging. As a result, ammonia is stored in liquid state at low temperatures or under high pressure.
Recent studies have demonstrated that ammonia can be stored at room temperature and under ordinary pressure by incorporating it into the pores of porous compounds, such as activated carbon, zeolites, and organometallic structures. However, under these conditions, corrosive ammonia is stored as it is, which raises safety concerns.
The perovskite compounds analyzed by the joint research group exhibit one-dimensional (1D; columnar), two-dimensional (2D; layered), or three-dimensional (3D) structures. Moreover, upon exposure to water vapor or chemical vapors, the perovskites incorporate the vapors and undergo structural changes.
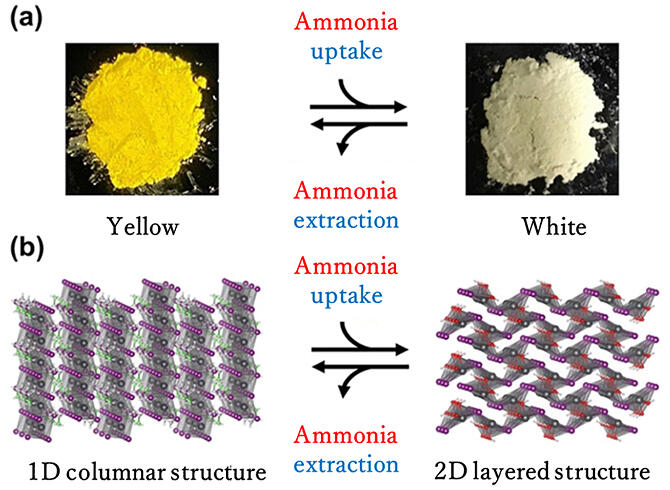
Because of the structural changes that they undergo and molecular-sized space of ∼ 0.9 nm between the columnar structures of the 1D perovskite compounds, the researchers induced a chemical reaction involving a structural change by bringing ammonia in close proximity with the perovskites.
First, the simplest structure of the 1D columnar perovskite compound (CH3CH2NH3PbI3) was chemically synthesized. The compound is a yellow crystal with a smooth and poreless surface. Upon exposure to ammonia vapor at room temperature and under ordinary pressure, the perovskite compound is converted to white crystals (Pb(OH)I). X-ray diffraction analysis revealed that the white crystals exhibited a 2D layered structure.
Researchers hypothesized that the structural change was induced by the chemical reaction of the perovskite compound with ammonia as the trigger. The chemical reaction was investigated, and it was determined that ammonia was chemically converted and existed as two types of nitrogen compounds (i.e., CH3CH2NH2 and NH4I) on the surface of the 2D layered structure.
Upon heating to 50 ℃ under vacuum, the nitrogen compounds stored on the surface of the 2D layered structure reversely reacted to form ammonia. Furthermore, the crystals changed color from white to yellow, and the structure reverted to a 1D columnar structure. The perovskite compounds that revert to a 1D columnar structure can be reused, allowing for repeated storage and retrieval of ammonia.
Compared to porous compounds, perovskite compounds are a safer storage medium because they store nitrogen compounds, which are less corrosive than ammonia. The temperature at which ammonia is retrieved is at least 100 ℃ lower than that of porous compounds, making the handling of corrosive gases easier. This achievement promotes the effective use of ammonia and provides critical guidance for the achievement of a decarbonized society.
Porous compounds non-selectively adsorb various substances, such as gases, molecules, and ions. Conversely, the perovskite compound synthesized in this study reacts only with ammonia and is therefore thought to be able to selectively store ammonia from gas mixtures.
Journal Information
Publication: Journal of the American Chemical Society
Title: Chemical Storage of Ammonia through Dynamic Structural Transformation of a Hybrid Perovskite Compound
DOI: 10.1021/jacs.3c04181
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




