Developing a medication for preventing dementia at a stage before amyloid-β deposition induces neuronal death could be possible. A research group led by Associate Professor Hisashi Shirakawa of the Graduate School of Pharmaceutical Sciences at Kyoto University and Assistant Professor Masashi Kakae of the School of Pharmaceutical Sciences at Wakayama Medical University (doctoral student of the Graduate School of Pharmaceutical Sciences at Kyoto University at the time of research) demonstrated that regeneration of the myelin sheath in the brain could be promoted by stimulating the transient receptor potential ankyrin 1 (TRPA1) channel, a thermal and chemical sensor. According to Shirakawa, "We hope that our findings will aid the development of a therapeutic medication, such as a drug that stimulates the TRPA1 channel or promotes the release of leukemia inhibitory factor (LIF; located downstream of the channel), that can be administered at a stage of mild cognitive impairment before neuronal death occurs." The study was published in Science Advances.
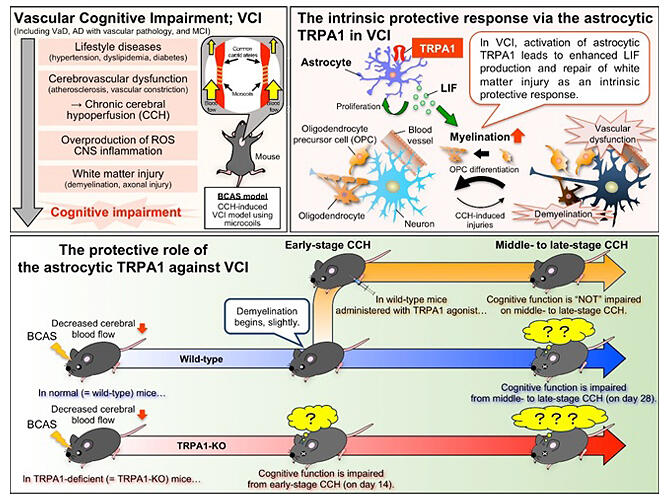
Flow of factors leading to vascular cognitive impairment (VCI) in mice; the astrocytic TRPA1 channel mediates an intrinsic protective response to VCI via leukemia inhibitory factor (LIF) production.
(Lower half)
Comparison in protective response to VCI between TRPA1-deficient and wild-type mice, with and without TRPA1 agonist administration
Provided by KyotoU/Hisashi Shirakawa
In recent years, studies have reported that more than 80 % of older patients with Alzheimer's disease have co-occurring cerebrovascular impairment, which led to the mainstream assumption that most patients with dementia have coexisting pathologies of Alzheimer's disease and cerebrovascular disease.
Vascular cognitive impairment (VCI), which refers to cognitive decline caused by cerebrovascular impairment, includes symptoms of vascular dementia, preclinical cognitive impairment, Alzheimer-type dementia with vascular pathology, and mild cognitive impairment. Many scientific articles have reported that a decrease in cerebral blood flow (known as cerebral hypoperfusion) occurs before the onset of these VCI symptoms or at the early stage of their manifestation.
In a longitudinal study that followed up more than 400 participants aged 75 years and older, 122 participants developed dementia within 6-7 years, and 65 developed Alzheimer's disease. The incidences were twice as high among hypotensive participants (i.e., individuals with low blood pressure). In another longitudinal study of 1270 participants without dementia (aged 75-101 years), the relative risk of Alzheimer's disease was significantly higher among the hypotensive individuals (relative risk: 1.7). These data suggest that, among older adults aged 75 and older, those with hypotension have a higher risk of developing dementia.
Atrophy (i.e., the wasting away) of the brain, especially of the white matter, is observed among patients with Alzheimer's disease. Oligodendrocytes, which mainly comprise white matter, form a myelin sheath that protects nerve cells, prevent crosstalk of electrical signals flowing to nerve cells, and aids in efficient signal delivery. White matter damage is caused by cerebral hypoperfusion.
In summary, the following mechanism can be assumed: Aging and lifestyle-related diseases cause arteriosclerosis and vascular hypofunction, leading to cerebral hypoperfusion, white matter damage, and neuronal death, which promote cognitive decline.
In the Kyoto University study, the research group analyzed the role of the TRPA1 channel in VCI using a mouse model of bilateral common carotid artery stenosis (BCAS). A microcoil was applied to the common carotid artery to slowly induce cerebral hypoperfusion in the mouse to establish the model.
Two objects were placed in the cage housing the BCAS mice. After 6 hours, one of the objects was replaced with a new object. After 10 minutes, the wild-type BCAS mice were observed to be interacting more with the novel object, indicating a learning effect. However, TRPA1-knockout BCAS mice did not show a learning effect and interacted equally with the novel and original objects. White matter damage due to cerebral hypoperfusion was observed only on days 14 and 28 in the wild-type BCAS mice. In contrast, such damage occurred earlier in the TRPA1-knockout BCAS mice.
When wild-type BCAS mice were continuously administered cinnamaldehyde (the main component of cinnamon) intraperitoneally for 10 days from day 15 onward to stimulate TRPA1, neither white matter damage nor cognitive impairment was observed. The research group performed a comprehensive analysis of astrocyte-related gene expression after TRPA1 stimulation and observed an increase in the expression of several genes, including the one encoding LIF. The analysis revealed that the increased astrocyte expression of LIF in the wild-type BCAS mice was reduced in the TRPA1-knockout BCAS mice. Furthermore, in cell culture experiments, transferring TRPA1-stimulated astrocyte medium to oligodendrocyte precursor cells (OPCs) enhanced OPC myelination.
These results showed that by promoting myelination through LIF production, TRPA1 in astrocytes plays a protective role in preventing chronic cerebral hypoperfusion from causing white matter damage and subsequent early onset of cognitive impairment.
Shirakawa stated, "Our findings suggest that in VCI, the TRPA1 molecules in astrocytes promote LIF secretion and repair white matter damage (demyelination) that causes cognitive impairment. In our study, neuronal death did not occur during the experimental period (up to postoperative day 28). If myelin sheath repair can be promoted by stimulating TRPA1, it may be possible to prevent dementia itself before the onset of Alzheimer's disease, which involves neuronal death. We would like to further investigate the involvement of related molecules in detail using the current model and other animal models of dementia to obtain a complete picture of the onset of dementia and the defense mechanisms against it."
Journal Information
Publication: Science Advances
Title: The astrocytic TRPA1 channel mediates an intrinsic protective response to vascular cognitive impairment via LIF production
DOI: 10.1126/sciadv.adh0102
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




