The research group led by Graduate Student Akari Nakamura, Assistant Professor Seiichiro Sakai, and Professor Takashi Shichita in the Department of Neuroinflammation and Repair, Medical Research Institute, Tokyo Medical and Dental University (TMDU) collaborated with the University of Tokyo, Tokyo Metropolitan Institute of Medical Science (TMIMS), and Keio University to successfully clarify the mechanism by which fatty acid metabolites produced in the brain after cerebral infarction repair surviving neurons. The researchers confirmed increased levels of the dihomo-γ-linolenic acid lipid and its metabolite 15-HETrE, which played undetermined roles in the brain after cerebral infarction in mice.
Moreover, the group demonstrated that the administration of 15-HETrE improved neurological symptoms in the mouse model of cerebral infarction. This compound is expected to be used as a new therapeutic agent to be administered 24 hours after the onset of cerebral infarction, a condition for which no effective therapeutic drug exists. The results were published in the July 24th, 2023 issue of the international academic journal Neuron.
Provided by TMDU
Cerebral infarction is a disease in which blood vessels in the brain become clogged and necrosis of brain tissue occurs, which accounts for 80% of strokes. In the treatment of cerebral infarction, the degree of damage to brain function varies greatly depending on the time from onset to treatment. While this damage can be suppressed if treatment is administered within 4−5 hours, it is difficult to suppress after 24 hours. Since lost functions can be restored to some extent after 2−3 months of rehabilitation, it is thought that the ability to repair nerve cells is acquired and neural circuits are reconstructed during this period.
However, before the present study, how neurons that survived the cerebral infarction acquired this restorative ability was unknown.
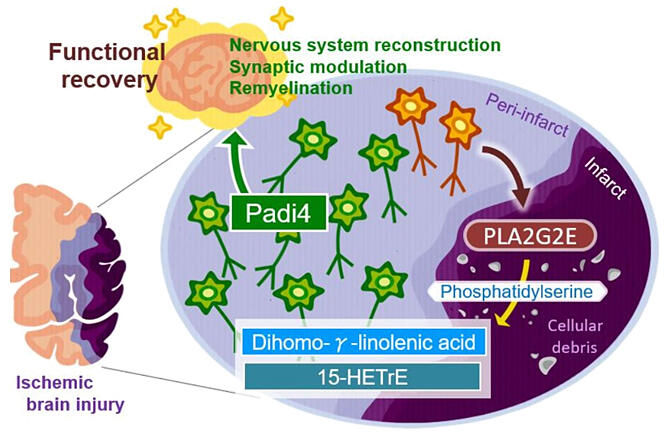
The research group used a mouse model of cerebral infarction caused by surgical treatment. The researchers extracted lipids from the tissue around the cerebral infarction lesion up to 1 week after the onset and compared the type and amount of lipids to those in unaffected mice. Lipid metabolism changed after cerebral infarction.
The amount of unsaturated fatty acids increased 3−6 days after the onset of cerebral infarction. Among these unsaturated fatty acids, an increase in the levels of dihomo-γ-linolenic acid was confirmed. The researchers clarified that the lipid was cleaved from phosphatidylserine by the enzyme PLA2 and confirmed that the prognosis after cerebral infarction was exacerbated in PLA2-deficient mice.
Furthermore, mice lacking this enzyme showed reduced expression of genes involved in nerve repair, as well as expanded necrotic areas. Among the genes associated with nerve repair, the expression of citrullination enzyme PADI4 was most markedly decreased. PADI4-deficient mice demonstrated exacerbation of neurological symptoms and expansion of necrotic areas after cerebral infarction.
PADI4 reportedly citrullinates histone proteins, loosening chromatin structure and activating gene transcription. Therefore, the researchers examined the involvement of PADI4 in the activation of repair function in neurons after cerebral infarction. They found that PADI4 citrullinated histones were associated with the transcriptional regulatory regions of genes that act in nerve repair, thus activating the genes and their function.
Next-generation sequencing examination of the gene expression levels of single cells isolated from the area surrounding the cerebral infarction of PADI4-deficient mice showed that the nerve-repairing gene was not expressed but was expressed in model mice without this deficiency.
Furthermore, the researchers identified 15-HETrE, a metabolic lipid of dihomo-γ-linolenic acid, as a factor that strongly induced PADI4. The administration of this lipid to mice with cerebral infarction resulted in increased expression of PADI4 and genes involved in nerve repair in neurons around the cerebral infarction, as well as amelioration of the symptoms of cerebral infarction and reduction of necrotic areas.
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




