A research group consisting of Team Leader Takashi Tsuji, Senior Scientist Makoto Takeo, and Visiting Scientist Miho Ogawa of the Laboratory for Organ Regeneration at RIKEN Center for Biosystems Dynamics Research announced that they have clarified a new biological rhythm using a hair morphogenesis model. They analyzed the mechanism of a new biological rhythm from a special model of hair morphogenesis and micro-niches created by dermal papilla cells in hair follicles. Dermal papilla cells are stem cells that maintain their properties in vivo. The research team clarified that hair follicle-specific rhythms are generated by the spatiotemporal changes in hair matrix cells, as well as the micro-environmental changes necessary for hair growth. The results were published in the international academic journal Nature Communications on August 4.
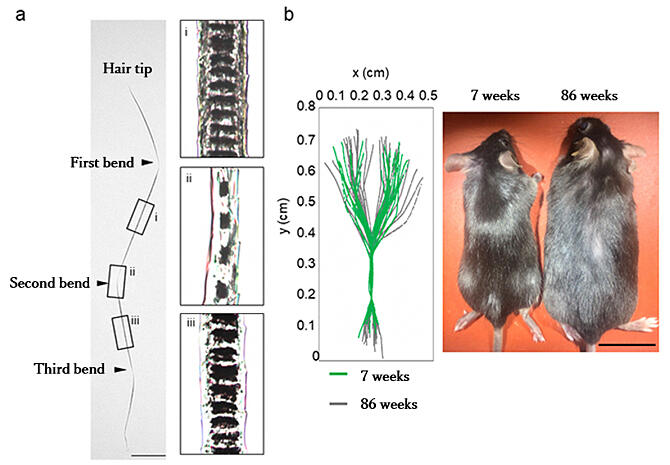
b) 2D plots (left) and stereographs (right) of hair shafts of 7-week-old and 86-week-old mice. 7-week-old mice show bend points around 0.2, 0.35, and 0.5 cm on the y axis, with intervals ranging from 1.5 to 1.8 mm and an almost constant inflection angle of about 160 degrees (green line in the left panel). In 86-week-old mice on the other hand, these values are disrupted, and the morphology of zigzag hairs tends to vary (gray line in the left figure). In addition, the 7-week-old mice have shiny fur, while the 86-week-old mice do not. Scale bar is 5 cm.
Provided by RIKEN
Mammalian hair is produced in the hair bulb that is present at the base of a hair follicle. Hair follicles undergo cyclical involution and regeneration throughout life, and hair is replaced in accordance with this cycle. Mice have different types of hair that are morphologically distinct, and the zigzag-type hair, which accounts for 70% of their hair, always grows in a pattern in which it bends left and right three times at fixed positions.
The research team used mouse zigzag hair as the model for their study, particularly in the bend formation process, to investigate the mechanism by which the biological rhythm of postnatal morphogenesis is maintained.
The bend formation period in zigzag hair was nearly constant in young adult mice but was variable in old mice. They found that bends formed once about every three days in a biological rhythm, and that this rhythm was disturbed by aging.
To clarify the cellular mechanism of bend formation, they analyzed the cellular dynamics of dermal papilla cells and of hair matrix cells that interacted with dermal papilla cells in the hair bulb, during the second bend formation. Dermal papilla cells are composed of four types of cell groups (clusters) of C1 to C4, each creating different microenvironments, called micro-niches.
During the three-day bend formation process, a growth-arrested region was formed once a day in a part of the hair matrix cells in a bilateral asymmetrical manner, always on the same side of the dermal papilla. Within the three-day bend formation, the growth-arrested area faced only toward the C2 cluster of cells of the papilla micro-niche on days 1 and 2. This area then faced toward both the C2 and C3 clusters on day three when the bend was formed.
In addition, the growth-arrested region of the hair matrix, which formed in contact with the C2 and C3 clusters, moved to the upper part of the hair follicle as the hair shaft grew and enlarged; thereby crushing and transforming the hair shaft. In this way, the bend was formed. These results suggested the new concept that cell dynamics is involved in biological rhythm formation.
To clarify the molecular mechanism of bend formation, they analyzed gene expression changes in the hair bulb during the bend formation process. They found considerable increases in the pleiotrophin gene (Ptn), a growth factor (secreted factor) that is involved in regulating cell behavior, as well as interactions between the epithelium and the mesenchymal tissues in multiple organs. They then revealed that expression of a certain ALF transcription elongation-factor 3 gene (Aff3), which encodes a factor involved in multiple biological processes, was also notably increased during the bend formation stage. On the 2nd and 3rd day of the bend formation period, Ptn was expressed in the proliferation-arrested region of hair matrix cells, while Aff3 was strongly expressed in the C3 dermal papilla micro-niche.
To investigate the functions of Ptn and Aff3 in bend formation, the group reconstituted hair follicles (regenerated hair follicle primordium) with cells in which the expression of these genes was artificially suppressed or they were artificially overexpressed, and then examined the influence on the zigzag hair inflection. The research team found that Ptn plays a role in the formation of growth-arrested regions of hair matrix cells once a day, while Aff3 regulated micro-niche switching once every 3 days, thereby maintaining the proper rhythm for bend formation.
Aging disrupts morphogenetic patterns by disturbing the biological rhythm. Furthermore, it is conceivable that changes in hair quality in humans arise from such biological rhythm modulation. This indicated a possible contribution to aging medicine research. Moreover, it pointed toward a new approach to improve hair texture.
Tsuji described the result, "Hairs originally come from a fibrous aggregate of epithelial cells that becomes the hair shaft. At first, I thought that, if we clarified the mechanism of the division of hair matrix cells, or if we looked closely at the papilla cells that order proliferation, we might be able to solve the problem. But this turned out to be extremely difficult. Thanks to the researchers and technical staff who specialize in cell movement, I could unravel this rhythm and morphogenesis from cell movement. I believe that we have shed light on a possible initial idea for biological methods that could be used to improve hair quality and in anti-aging."
Journal Information
Publication: Nature Communications
Title: Cyclical dermal micro-niche switching governs the morphological infradian rhythm of mouse zigzag hair
DOI: 10.1038/s41467-023-39605-z
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




