Freezing of water-rich foods, medicines, and biological tissues leads to growth of ice crystals and destruction of cell membranes and organelles. Therefore, methods wherein the growth of ice crystals is inhibited by adding a cryoprotectant have been developed. However, the mechanism underlying the inhibition of the growth of ice crystals is unclear.
Principal Researcher Takayuki Kumada, Principal Researcher Hiroshi Nakagawa and Assistant Principal Researcher Yurina Sekine of the Materials Sciences Research Center at the Japan Atomic Energy Agency (JAEA), Assistant General Manager Kazuki Ohishi and other researchers of the Comprehensive Research Organization for Science and Society (CROSS), Professor Kiyoshi Kawai of the Graduate School of Integrated Life Sciences for Life at Hiroshima University succeeded for the first time in observing the unique shape of ice crystals immediately after their formation in an aqueous glucose solution. Spin-contrast modulated small-angle neutron scattering was used for the observation. The results were published in The Journal of Physical Chemistry Letters.
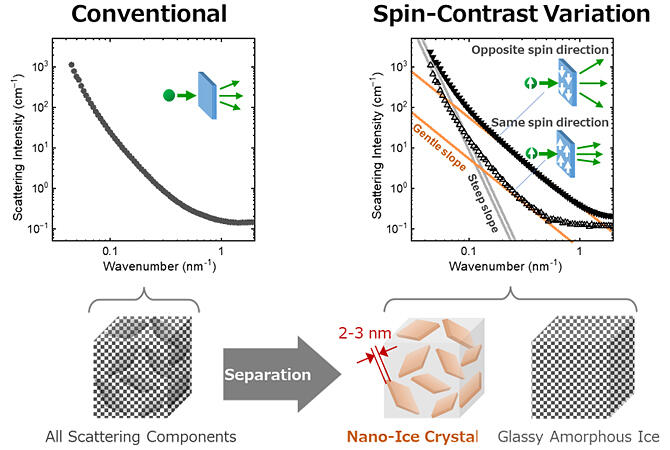
Provided by JAEA
The research group performed spin-contrast modulated small-angle neutron scattering experiments by integrating a hydrogen nuclear polarizer into the small-angle and high-angle neutron scattering system at the Japan Proton Accelerator Research Complex (J-PARC). Upon the addition of glucose, the ice crystals formed plates with a thickness of 2−3 nm that spread over tens of nanometers. This thickness is approximately equal to the critical diameter for ice crystal nuclei to initiate crystallization in supercooled water.
To date, sugars have been understood to inhibit the growth of ice crystals by binding (hydrating) to the surrounding water molecules. However, these results indicate that glucose not only hydrates the water molecules but also inhibits the growth of ice crystals. Thus, similarly to other cryoprotectants, sugars can also possibly bind strongly to specific faces of the ice crystals and suppress growth in that direction.
Kumada said, "In the future, we would like to clarify the mechanism by which various sugars and other cryoprotectants inhibit the growth of ice crystals. Additionally, by unraveling the functions of specific sugars produced in plants in cold regions, we expect to contribute to understanding the survival of plants in cold regions."
Journal Information
Publication: The Journal of Physical Chemistry Letters
Title: Polarized Neutrons Observed Nanometer-Thick Crystalline Ice Plates in Frozen Glucose Solution
DOI: 10.1021/acs.jpclett.3c01448
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




