A research group led by Researcher Yuto Kita (at the time of research) and Associate Professor Akitsu Hotta of the Center for iPS Cell Research and Application (CiRA) at Kyoto University announced that they have developed a dual CRISPR-Cas3 system that induces exon skipping in induced pluripotent stem (iPS) cells obtained from patients with Duchenne muscular dystrophy (DMD). With two CRISPR-Cas3 systems, it is possible to delete the wide exon 45−55 region present in the DMD gene at once. By applying this system to iPS cells established from patients, they confirmed that dystrophin protein was restored. The result is expected to lead to the development of treatments for DMD. The achievement was published in the August 15 issue of the international academic journal Stem Cell Reports.
Provided by Kyoto University
DMD is an orphan disease that causes atrophy of muscles throughout the body. It is caused by mutations in the gene coding for dystrophin, an important protein for maintaining muscle cells. This X-linked disease develops mainly in male infants and is the most common type of muscular dystrophy in children. There are approximately 2,000−3,000 patients afflicted with DMD in Japan, and the average lifespan is short (∼31 years).
The gene encoding dystrophin is relatively large, consisting of 79 exons. A mutation in any of these exons causes malfunction of the dystrophin protein and muscular dystrophy. The location of the mutation varies from patient to patient, and efforts have been made to develop methods to restore dystrophin for each mutation. For example, exon skipping has attracted attention, and nucleic acid drugs that skip exon 53 are being sold in Japan and the United States of America by agencies such as the National Center of Neurology and Psychiatry (NCNP).
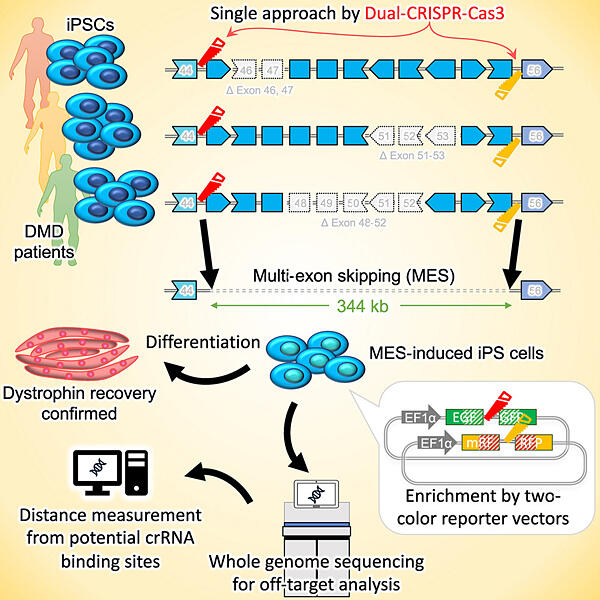
Approximately 60% of patients with DMD have mutations in exons 45−55 (spanning 344 kilobases). It has been reported that multi-exon skipping (MES), in which the region is skipped at once, may be induced to restore dystrophin in certain patients. Patients with deletions in this exon region are classified as having the mild Becker type of muscular dystrophy, with almost no muscular symptoms. In contrast, the existing popular CRISPR-Cas9 technique generates only microdeletions of a few bases, making it difficult to delete large areas.
The research group explored a technique that can delete exons 45−55 from both ends using two CRISPR-Cas3 systems, which edit a wider range of genomes than the existing CRISPR-Cas9 system. The new CRISPR-Cas3 system developed in Japan can delete 20−60 kilobases. Although the efficiency was still not sufficiently high, the Cas3 system was able to induce MES more efficiently than Cas9. They also developed a method to enrich the cell population in which the exon region was successfully deleted. With the same system, MES induction was subsequently achieved in iPS cells derived from the cells of three patients with DMD that contained different genetic mutations in the same range.
As a result, the research group ensured that iPS cells derived from any patient would have the same 344-kilobase region deleted. It was confirmed that these patient-derived cells, which were unable to produce dystrophin before, were able to produce the protein in skeletal muscle after MES induction. Through whole-genome analysis, they also confirmed that MES-induced cells did not have unintended deletions (off-target effects). Cells generated using this system are being used to repair muscle lost due to disease progression.
Hotta said, "By using the system, we can achieve the recovery of dystrophin even in 100 patients with the same method. We aim to create a very simple and low-cost production line for therapeutic cells. As a future challenge, we believe that if a technology for delivering CRISPR-Cas3 into the body is developed, we can apply it to in vivo gene therapy to restore dystrophin in the patient's body."
Journal Information
Publication: Stem Cell Reports
Title: Dual CRISPR-Cas3 system for inducing multi-exon skipping in DMD patient-derived iPSCs
DOI: 10.1016/j.stemcr.2023.07.007
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




