A research group led by Senior Researcher Takao Yoshida of the Research Institute for Global Change, together with the Research Institute for Marine Resources Utilization and the Institute for Extra-cutting-edge Science and Technology Avant-garde Research of the Japan Agency for Marine-Earth Science and Technology (JAMSTEC), in collaboration with the School of Marine Biosciences of Kitasato University, the School of Medical Sciences of Fukui University, the Institute of Low Temperature Science of Hokkaido University, and Fukuoka Women's University, studied the intracellular symbiotic system in the bivalve mussel Bathymodiolus japonicus, which lives in areas of methane upwelling in the deep sea. The research group announced that they were the first in the world to discover that the mechanistic target of rapamycin complex 1 (mTORC1), a regulatory protein complex that integrates intracellular nutrient-environment signals from the host animal, regulates the maintenance and digestion of symbiotic bacteria. Additionally, they have unraveled the mechanism of this regulatory process.
They achieved this by collecting B. japonicus individuals from the deep-sea region off Hatsushima Island in Sagami Bay (at ∼900 m depth) and examining them using isotope ratio analysis, gene expression analysis, rearing experiments, and microscopic observations. The results are expected to lead to the clarification of the mechanisms through which intracellular symbiosis is established and maintained and were published in the 23 Aug 2023 issue of Science Advances.
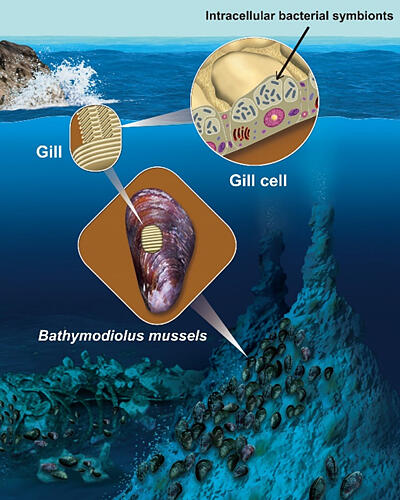
Most B. japonicus have an intracellular symbiotic relationship with chemosynthetic bacteria in their gill tissues. Chemosynthetic bacteria are taken in from the external environment and form a symbiotic relationship. (Figure created by Nariyuki Yoshihara)
Provided by JAMSTEC
Deep-sea hydrothermal and methane upwelling areas have been identified as important candidates as offshore-marine protected areas for biodiversity conservation and have been shown to contain valuable resources, such as minerals (including rare metals) and methane hydrate. Understanding the ecology of the organisms living in the hydrothermal and methane upwelling areas is important for balancing resource development and biodiversity conservation.
Although B. japonicus individuals have mouths, their digestive tracts are shorter than those of related mussel species. Moreover, they do not feed on their own food, relying instead on the organic matter produced by symbiotic bacteria that live in their cells. How they acquire and select specific chemosynthetic bacteria as symbionts from the numerous microorganisms in the environment and maintain them in their cells remained a mystery.
In this study, the research group found that the membrane enveloping the symbiotic bacteria is phagocytic, with the presence of digestion-related proteins involved in phagocytosis on its surface. Additionally, mTORC1, a protein complex that senses intracellular nutrients and environmental conditions and integrates and regulates cellular nutrient-environment signaling, was also found at the membrane surface. mTORC1 is a ubiquitous eukaryotic protein complex that is believed to act as a command post for monitoring the intracellular nutrient status and regulating multiple cellular functions.
During the long-term culture of B. japonicus without the addition of methane, symbiotic bacteria in the phagosome, where mTORC1 is present, are degraded by digestive enzymes. However, the researchers found that the symbiotic bacteria remained undigested when the mussels were grown in the presence of an inhibitor of mTORC1. B. japonicus rapidly and indiscriminately incorporates microorganisms into its gill cells through phagocytosis and then digests all of them except the symbiotic bacteria. The symbiotic bacteria in the phagosome were observed to be recognized and maintained by the cell through the mTORC1 detection of amino acids and other organic matter that they secrete.
mTORC1, acting as a command post in the regulation of nutrient signal integration in eukaryotic cells, has been revealed to have a novel function in the maintenance and degradation of symbiotic bacteria in the intracellular symbiotic system of the mussel. Intracellular symbiosis is a phenomenon that has driven the evolution of eukaryotes. mTORC1 may also regulate intracellular symbiosis in the cellular systems of other organisms, such as insects.
Yoshida stated, "Bathymodiolus japonicus is difficult to culture because it lives in deep water and must be collected on a voyage and immediately experimented with. The opportunity to collect these mussels is limited to once a year. It has been 13 years since we began our research. Finally, five years ago, we obtained the result that mTORC1 is involved in intracellular symbiosis. It was a long road, but it culminated in a photograph of a colony of Bathymodiolus japonicus on the cover of a magazine. We will further investigate the role of mTORC1 in intracellular symbiosis in the future."
Journal Information
Publication: Science Advances
Title: mTORC1 regulates phagosome digestion of symbiotic bacteria for intracellular nutritional symbiosis in a deep-sea mussel
DOI: 10.1126/sciadv.adg8364
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




