Assistant Principal Researcher Hirokazu Ueta and Group Leader Katsuyuki Fukutani (Professor of the Institute of Industrial Science (IIS) at the University of Tokyo) of the Research Group for Surface and Interface Science in the Advanced Science Research Center (ASRC) at the Japan Atomic Energy Agency (JAEA) announced that they have clarified the heat transfer process between gas and solid. They constructed an experimental system to investigate the state change of molecular hydrogen and discovered, for the first time, that rotational energy is transferred to electrons and lattice vibrations on a metal surface. This achievement is expected to enable flexible control of heat transfer. The results were published in the August 20, 2023 issue of the Journal of Physical Chemistry Letters.
Provided by JAEA
Energy (heat) transfer between gas and a solid surface is a crucial aspect in the control and utilization of heat. There are three types of molecular motion: 'translation,' 'vibration,' and 'rotation.' The mechanisms of translational-energy and vibrational-energy transfers in molecular motion have been extensively investigated. By contrast, rotational-energy transfer has been unclear owing to its low energy content; this has hindered the construction of experimental systems. In this study, the researchers constructed an experimental system by combining two types of lasers and molecular beams.
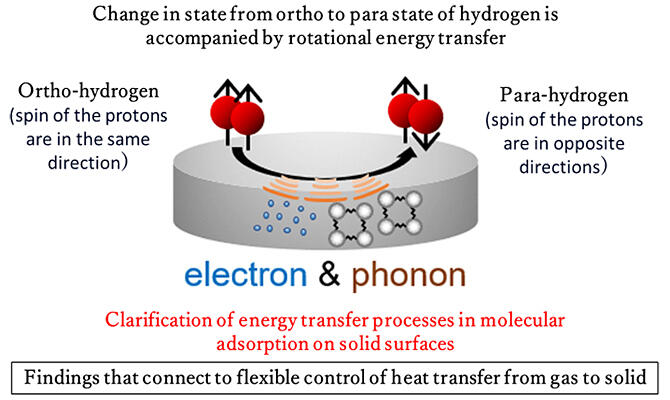
In regard to molecular hydrogen, there are two types of spin isomers—ortho-hydrogen and para-hydrogen—each with different rotational energy states. To convert ortho-hydrogen into para-hydrogen, they focused on the release of rotational energy upon the reversal of spin direction and examined the state change.
In this experiment, molecular hydrogen was adsorbed on a palladium surface, and the rotational energy of hydrogen was examined. The rotational energy of ortho-hydrogen was found to be approximately 10 meV higher than that of para-hydrogen. The rate of ortho-to-para conversion was examined across surface temperatures ranging from -232 ℃ to -213 ℃. This conversion rate increased with increasing surface temperature.
They devised a model for rotational-energy transfer based on the assumption that electron involvement is necessary for the state change of ortho-to-para conversion. It was revealed that hydrogen energy is transferred to electrons and lattice vibrations on the metal surface. Given that the magnitude of the lattice vibration is derived from the constituent elements and structure, the possibility of controlling heat transfer through the control of lattice vibration was demonstrated.
Further verification will continue through experiments involving other metals with different lattice vibrations. These findings are expected to applicable in the design of alloys and other materials.
Journal Information
Publication: Journal of Physical Chemistry Letters
Title: Rotational-Energy Transfer in H2 Ortho−Para Conversion on a Metal Surface: Interplay between Electron and Phonon Systems
DOI: 10.1021/acs.jpclett.3c01209
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




