A research group led by Researcher Satoshi Takebayashi and his colleagues of the Science and Technology Group (STG) at the Okinawa Institute of Science and Technology Graduate University (OIST), and Professor Kazunobu Sato of the Graduate School of Science and his colleagues at Osaka Metropolitan University, in collaboration with Justus Liebig University Giessen, Germany, and the Russian Academy of Sciences, announced the successful development of a new metallocene compound, which are organometallic compounds often used as catalysts.
To create this structure, two additional electrons were added to the 19-electron metallocene structure, leading to a 21-electron metallocene compound. The synthesized compound was found to be stable in both the solution and solid states and could be stored for prolonged periods. This development could be a major breakthrough leading to the future creation of new materials in the medical and energy fields. The results have been published in the 05 September 2023 issue of Nature Communications.
Provided by OIST
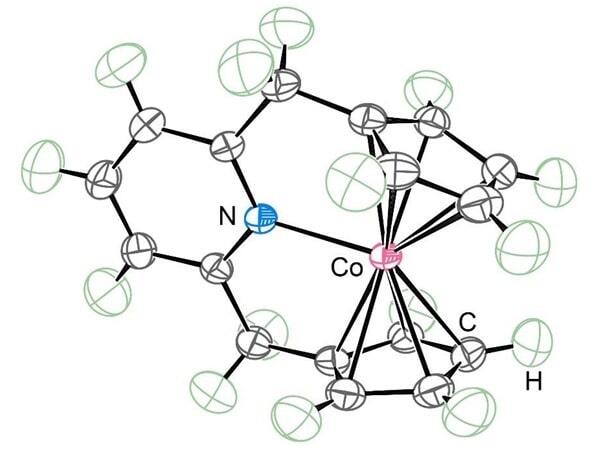
Organometallic compounds are molecules composed of metal atoms and organic molecules, and over the years, these molecules have notably contributed to developments in the field of chemistry owing to their chemical-reaction-promoting abilities. Metallocene, a type of an organometallic compound, has a special sandwich structure and a wide range of applications. Its chemical structure can accommodate varying numbers of electrons, and it can form complexes with as many as 20 electrons. The most stable of these is known to be the 18-electron structure.
This structure can be used for various applications, including polymer production, in glucometers for blood glucose measurements, in perovskite solar cells, and as a catalyst. However, when the number of electrons exceeds 18, the chemical bonds are elongated or broken, leading to structural changes. As a result of this, the synthesis of 21-electron metallocenes has long been considered impossible. Particularly, demonstrating that nitrogen can bond with cobalt without altering the sandwich structure has proven to be the biggest hurdle in this development.
The sandwich structures of metallocenes can be easily altered, meaning that the research group needed to assemble a strong team of researchers with different areas of expertise to rigorously prove that the metallocene was correctly bonded to all its neighboring carbon atoms and that nitrogen atoms were bonded to cobalt atoms; eventually, the team succeeded in proving that these elements were bonded to each other. This new metallocene compound has the potential to create new materials that can be used not only as catalysts but also in medical and energy applications.
Takebayashi commented, "Although this study focused on the basic molecular structures and physical properties of metallocene, this new compound has the potential to create new materials with prospective applications in catalysis, medicine, energy, and other fields. This may help solve important global issues and improve our quality of life."
Journal Information
Publication: Nature Communications
Title: Synthesis and characterization of a formal 21-electron cobaltocene derivative
DOI: 10.1038/s41467-023-40557-7
OIST English Press Release: https://www.oist.jp/news-center/news/2023/9/5/scientists-synthesize-new-organometallic-sandwich-compound-capable-holding-more-electrons
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




