Lecturer Toshimitsu Suzuki and Professor Kazuhiro Yamakawa of the Institute of Brain Science (IBS) at the Graduate School of Medical Sciences of Nagoya City University, Professor Tsuyoshi Miyakawa of the Institute for Comprehensive Medical Science (ICMS) at Fujita Health University, and Professor Hiroaki Mizukami of the Center for Molecular Medicine at Jichi Medical University collaborated to create mice with a homozygous deletion of the Scn2a gene in the medial prefrontal cortex (mPFC) or ventral tegmental area (VTA) of the brain, which are regions associated with schizophrenia. Their subsequent detailed analysis revealed that Scn2a deficiency in the mPFC induced decreased locomotor activity, increased anxiety-like behavior, and reduced the prepulse inhibition (PPI) of the auditory startle response, whereas Scn2a deficiency in the VTA increased PPI. Other behavioral tests (number of approaches, amount of locomotor activity, and anxiety-like behavior in these mice) revealed no changes associated with Scn2a deficiency.
This achievement improves understanding of neural circuit disorders that cause schizophrenia and neurodevelopmental disorders associated with Scn2a gene mutations and leads to the development of treatments for such disorders. The results were published in the scientific journal Molecular Neurobiology.
Provided by Nagoya City University Hospital
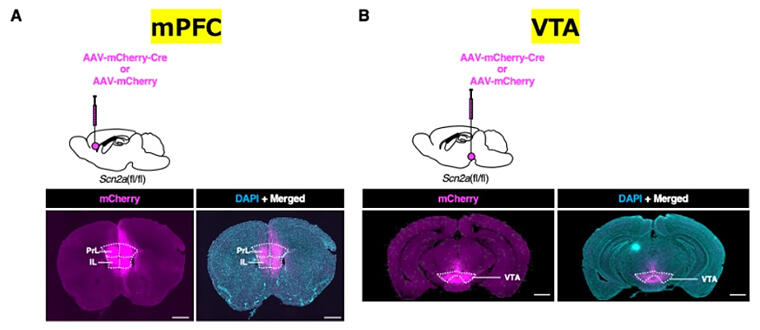
Mutations in Scn2a gene, which encodes the voltage-gated sodium channel α2 subunit (Nav1.2), widely occur in epilepsy and neurodevelopmental disorders, including autism spectrum disorder, intellectual disability, and schizophrenia. However, the details pertaining to the brain regions and neural circuits responsible for the onset of the behavioral disorders associated with schizophrenia remain unclear. The research group used an adeno-associated virus (AAV) vector to generate mice homozygous-deficient in the Scn2a gene in the mPFC or VTA regions of the brain.
Using these mice, the researchers first performed a three-chamber social-approach test to evaluate the mice's social behavior toward others, as well as their memory ability. In an experimental room divided into three parts, in which the mice could freely move, the researchers placed two cages with and without an unfamiliar mouse. The examining mouse was placed in the third central cage. The researchers compared the times that the examining mouse spent around the cages with and without the unfamiliar mouse. The results revealed that Scn2a deficiency in mPFC but not the VTA resulted in an increased approaching time to the unfamiliar mouse.
The researchers subsequently conducted an open field test to observe the amount of spontaneous activity in the Scn2a-deficient mice. In this test, two mice were separately placed in new and unfamiliar rooms, and their behaviors were observed for 120 minutes. The experiment revealed that Scn2a deficiency in mPFC decreased locomotor activity and increased anxiety-like behavior, whereas Scn2a deficiency in VTA did not have these effects. These results contradict those previously observed in Scn2a-knockout mice, suggesting that brain regions other than the mPFC and VTA likely play a critical role in these behavioral defects.
The research team further conducted the PPI test, a psychophysiological indicator of schizophrenia. Listening to a soft sound before hearing a loud sound suppresses the startle response, inducing auditory startle-response PPI. The PPI test revealed that Scn2a deficiency in the mPFC lowered PPI, whereas deficiency in the VTA increased it.
These results demonstrated the opposite effects on PPI of Scn2a deficiency in the mPFC and VTA regions. Although it is widely established that Scn2a gene mutations cause schizophrenia, mice with systemic heterozygous Scn2a knockout did not show decreased PPI. However, although the results of the present study showed that decreased mPFC function due to Scn2a deficiency caused a decrease in PPI, this change is probably canceled out in systemic heterozygous Scn2a knockout-mice due to the opposite effects on PPI of VTA deficiency on other brain regions.
The research group combined these findings with those of previously research involving animal models of schizophrenia and proposed a PPI neural circuit model for mice lacking Scn2a in the mPFC or VTA. The results of the present study indicate that the mPFC is an important brain region in schizophrenia caused by Scn2a gene mutations and also reveal the counteracting effect of deficiency in the VTA on PPI.
Journal Information
Publication: Molecular Neurobiology
Title: Inversed Effects of Nav1.2 Deficiency at Medial Prefrontal Cortex and Ventral Tegmental Area for Prepulse Inhibition in Acoustic Startle Response
DOI: 10.1007/s12035-023-03610-6
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




