Researcher Yasunori Enomoto (Visiting Researcher at the time of research; currently Assistant Professor, Department of Regenerative and Infectious Pathology, Hamamatsu University School of Medicine) and Team Leader Mitsuru Morimoto of the Laboratory for Lung Development and Regeneration at RIKEN Center for Biosystems Dynamics Research, and Lecturer Tatsuya Nagano of the Division of Respiratory Medicine at Kobe University Hospital announced that they have successfully clarified the earliest phenomenon that causes pulmonary fibrosis through their collaborative work by recreating mini alveoli on culture dishes.
The researchers prepared alveolar organoids (i.e., pseudo-alveolar tissue) following the three-dimensional culturing of alveolar epithelial cells. The analysis revealed that tissue stem cells composed of type II alveolar epithelial cells (AT2 cells) attained a special fibrosis-inducing state upon senescence (i.e., aging-related cell deterioration). The findings will help in unraveling the pathology of intractable idiopathic pulmonary fibrosis and in the development of new therapeutic drugs (idiopathic means a disease with no known cause, whereas intractable means hard to control or deal with). Their achievement was published online in the 31 August 2023 issue of the scientific journal Nature Communications.
Provided by RIKEN
Pulmonary fibrosis develops when lung fibroblasts differentiate into myofibroblasts. These myofibroblasts induce the expression of abnormal proteins, such as collagen, and accumulate on the alveoli of lungs. This accumulation leads to fibrosis (i.e., tissue thickening or scarring) of alveolar wall and is life-threatening, as the respiratory function deteriorates upon its progression. Idiopathic pulmonary fibrosis is one of the most common but inscrutable diseases without an effective treatment. It is classified as an intractable disease in Japan.
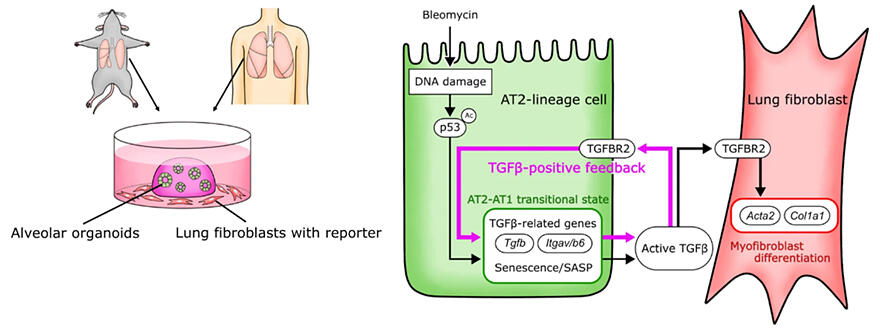
To reproduce pulmonary fibrosis in culture, the research group cultured mouse lung fibroblasts in an alveolar organoid culture medium containing bleomycin, a DNA-damaging chemical. A comprehensive analysis of the genes and proteins expressed in alveolar organoids revealed that the protein levels of p53 increased in the organoids that had undergone bleomycin-promoted DNA damage. p53, which is encoded by a tumor suppressor gene, is a typical biomarker of cell aging.
When alveolar organoids were treated with a drug that increases p53 signals, the surrounding lung fibroblasts differentiated into myofibroblasts, even in culture medium without bleomycin. A factor secreted in response to the p53 signal was found to be involved in fibrosis induction.
The researchers observed that when p53 signals increased in AT2 cells, the cells attained an 'AT2−AT1 transition-like state,' wherein they transiently expressed genes characteristically related to cell aging, corresponding to an intermediate state of cell differentiation (AT1 stands for type I alveolar epithelial cells.) In this cell state, the senescence-associated secretory phenotype (SASP) phenomenon caused proteins characteristically observed in senescent cells to be secreted.
The researchers examined the SASP protein group for the factor that induces myofibroblast formation and detected Transforming Growth Factor β (TGFβ). The TGFβ molecules secreted from AT2 cells to the surrounding area acted on the cells as an autocrine system (i.e., a system where the factor secreted from a cell acts on the factor-secreting cell itself), and causes a positive feedback situation that amplified the expression and activation of TGFβ.
Since the activation of TGFβ was sufficient for AT2 cells to change to an 'AT2−AT1 transition-like state,' the positive feedback of the factor was essential in these cells. The researchers concluded that this is a central mechanism of inflammation-independent pulmonary fibrosis. Furthermore, even when human lung cells were examined, the positive feedback of TGFβ was observed, and the inflammation-independent induction of myofibroblasts was confirmed.
Enomoto said, "During research initiation, we spent ample time creating organoids and transgenic mice. However, thanks to the support of various people, including the staff at RIKEN, we managed to put the results together. In the future, I would like to develop my research with a slight shift toward the clinical side to discover how to realize a treatment specific to AT2 cells."
Journal Information
Publication: Nature Communications
Title: Autocrine TGF-β-positive feedback in profibrotic AT2-lineage cells plays a crucial role in non-inflammatory lung fibrogenesis
DOI: 10.1038/s41467-023-40617-y
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




