A research group led by Professor Takayoshi Suganami and Designated Associate Professor Michiko Itoh of the Research Institute of Environmental Medicine (RIEM) at Nagoya University collaborated with Associate Professor Atsushi Tamura of the Institute of Biomaterials and Bioengineering (IBB) at Tokyo Medical and Dental University (TMDU) in conducting research on nonalcoholic steatohepatitis (NASH). They revealed that cholesterol accumulation in macrophages promotes liver fibrosis. This discovery was based on the analysis conducted on NASH mouse models and human NASH samples. They also confirmed that liver fibrosis in the NASH mouse model can be suppressed through excretion of the accumulated cholesterol. The result is expected to lead to the development of a NASH treatment and was published in the September 19, 2023 issue of the Journal of Experimental Medicine.
Provided by Nagoya University
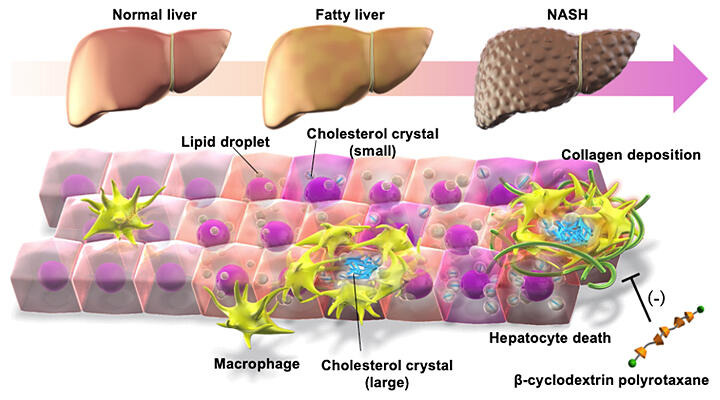
Obesity is increasing worldwide, and fatty liver is found in approximately 30% of people undergoing health checkups in Japan. Approximately 10−30% of fatty livers advance to NASH characterized by inflammation and fibrosis and progress further into hepatocellular carcinoma (liver cancer). However, the mechanism through which cytotoxic lipid accumulation in NASH worsens the disease condition has remained unclear.
The research group had previously used their original NASH mouse models to demonstrate that the disease develops through the accumulation of macrophages around dead hepatocytes (liver cells).
In this study, the research group discovered that cholesterol crystals formed within hepatocytes that had undergone cell death. They revealed that the cholesterol content was increased in CD11c-positive macrophages that accumulated around dead hepatocytes, and also that the lysosomes (organelles that break down biomolecules) were functionally impaired. This situation was also observed in human NASH liver.
Furthermore, they identified a unique supramolecule named beta-cyclodextrin polyrotaxane (βCD-PRX), which promotes the excretion of intracellular cholesterol in a lysosome-selective manner. When βCD-PRX was subcutaneously administered to a NASH mouse model continuously for 6 weeks, the cholesterol content of the CD11c-positive macrophages was selectively reduced, the profibrotic traits were suppressed, and the liver fibrosis was improved.
Analysis with cultured macrophages revealed that excessive cholesterol accumulation causes lysosomal damage and induces the expression of profibrotic factors through the family of transcription factor Es (TFEs, i.e., proteins that regulate gene expression) and others.
Journal Information
Publication: Journal of Experimental Medicine
Title: Lysosomal cholesterol overload in macrophages promotes liver fibrosis in a mouse model of NASH
DOI: 10.1084/jem.20220681
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




