Deputy Senior Researcher Takashi Tani and Researcher Yoshio Ishikawa of the Department of Radioecology at the Institute for Environmental Sciences (IES) have shown through the rearing of olive flounder in seawater containing deuterium (which behaves like tritium), that tritium in the seawater does not accumulate above the ambient concentration, even after binding to organic matter in the body, and it is discharged when the tritium concentration in the seawater decreases. Model simulations also confirmed these results. Some people have expressed concerns about the discharge of treated water off the coast of Fukushima Prefecture. However, their study results confirm that these fears can be allayed. The results were published in the journal Science of the Total Environment.
Provided by the Institute for Environmental Sciences
Tritium exists as water (tritiated water) when ingested into the body of an organism. However, some of the isotope is bound to the organic matter in the body. It has been pointed out that this organically bound tritium is more likely to remain in the body than tritiated water and may accumulate above ambient concentrations.
Recent studies have shown that organically bound tritium produced in the bodies of living organisms does not accumulate in the same way as tritiated water. However, although studies on fish have been conducted on freshwater species, experimental data on marine fish are scarce.
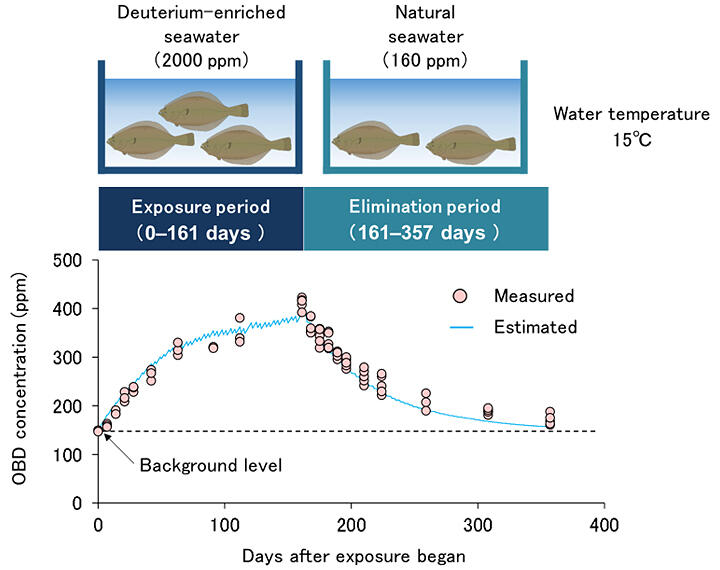
Like tritium, deuterium is an isotope of hydrogen that does not emit radiation. In this study, deuterium was ingested by olive flounder. In natural seawater, the ratio of deuterium to hydrogen is as low as approximately 0.016% (160 parts per million or ppm). In the experiment, olive flounders were reared for 161 days at 15℃ in seawater in which the deuterium concentration had been artificially increased to 2000 ppm (deuterated water). After 161 days, the concentration of organically bound deuterium (OBD) produced from the deuterated water in the body of the olive flounder was studied. Organically bound tritium and deuterium are not only produced from the water ingested by fish but also supplied by their feed.
In the study, researchers increased the concentration of deuterium in the seawater only, whereas the concentration in the feed was left at natural levels to decipher the movement of the isotope from the seawater. During the 161-day rearing period, the concentration of OBD in muscle, the edible part of the olive flounder, increased to approximately 400 ppm. This value was 20% of the deuterium concentration in the seawater used in the experiment.
When the olive flounders were returned to natural seawater, the concentration of OBD returned to near-natural levels by the end of the experiment. The model revealed for the first time that the biological half-life of organically bound tritium in olive flounder muscle is 133 days. The rates of formation and decay of OBD in olive flounder muscle were determined from the experimental data, and a model was developed that could reproduce the experimental data through calculation using these rates.
Using this model, a simulation calculation was performed for the case of continuous olive flounder rearing in seawater with a tritium concentration of 1500 becquerels per liter (the operational target for the discharge of water processed through the Advanced Liquid Processing System (ALPS)), with the tritium concentration in the feed kept at natural levels.
As an estimated result, the organically bound tritium produced from the seawater-derived tritiated water did not remain in the muscle and was discharged, and the concentration of organically bound tritium produced from tritiated water was diluted by the feed-derived organic matter. Moreover, equilibrium was reached at 114 becquerels per kilogram wet weight and did not increase further. If olive flounder reared under these simulation conditions is consumed as food for one year, the effective dose is estimated to be 0.0019 millisieverts per year, which is well below the 1 millisievert exposure limit for the general public.
The present study shows that neither tritiated water nor organically bound tritium accumulate in the muscle, the edible part of olive flounder. Additionally, the isotope is discharged through metabolism, and the concentration of organically bound tritium does not exceed the seawater isotope concentration to which the fish is exposed. The scientific evidence supports real-world observational data that tritium in seawater does not accumulate in marine fish.
Journal Information
Publication: Science of the Total Environment
Title: A deuterium tracer experiment for simulating accumulation and elimination of organically bound tritium in an edible flatfish, olive flounder
DOI: 10.1016/j.scitotenv.2023.166792
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




