A research group led by Distinguished Professor Susumu Kitagawa and Program-specific Assistant Professor Ken-ichi Otake of the Institute for Integrated Cell-Material Science (iCeMS) at Kyoto University has synthesized a porous material that only adsorbs carbon dioxide (CO2) from a variety of gases. This porous material internally adsorbs CO2 through a particular gateway but inhibits the adsorption of similar quantities of nine other gases including nitrogen, oxygen, and methane.
This study result is expected to facilitate the development of a new material that can efficiently separate and recover CO2 from exhaust gases containing various components emitted from factories and other facilities.
Provided by iCeMS, Kyoto University/Mindy Takamiya©.
The synthesized porous material is a type of porous coordination polymer (PCP), where organic molecules bind to metal ions, creating numerous pores at the molecular level. PCP is also called metal−organic framework (MOF). In 1997, Kitagawa demonstrated that a large amount of gas can be captured in the pores.
Activated carbon, silica gel, and zeolite are porous materials commonly used for adsorption, separation, and storage of gases. However, the pore sizes cannot be changed, and large molecules can only be forcefully adsorbed by applying pressure. By contrast, PCP can have a lattice structure that confines gases by replacing metal ions and organic ligands. Furthermore, the adsorption of gas molecules can alter certain lattice structures, thus advancing applied research for practical applications such as adsorbents and ion transport.
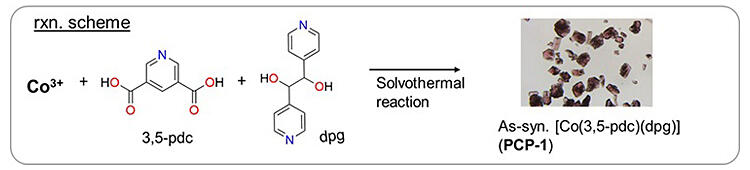
In 2021, the research group announced that they could separate CO2 from acetylene, gases with very similar molecular sizes and boiling points, using PCP made from cobalt ions and di(4-pyridyl) glycol (dpg). In this study, cobalt ion and pyridinedicarboxylic acid (pdc) were added to a solution of dpg and heated at 60 ℃ for 24 h to obtain pinkish-purple PCP crystals.
Provided by Program-specific Assistant Professor Ken-ichi Otake of the Institute for Integrated Cell-Material Sciences (iCeMS) at Kyoto University

Provided by Otake
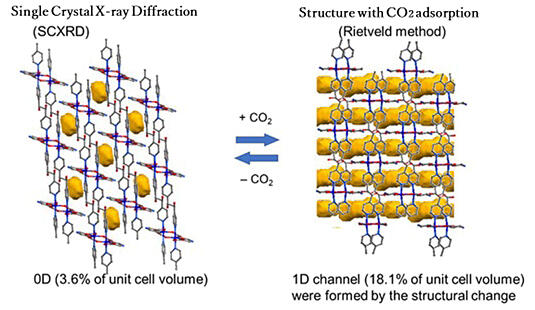
When the collected powdered crystals were combined with CO2 at -78 ℃ and 1 atm or at room temperature and approximately 6−7 atm, the lattice structure, resembling divided pockets, created a continuous pathway through the adsorption of CO2.
Provided by Otake
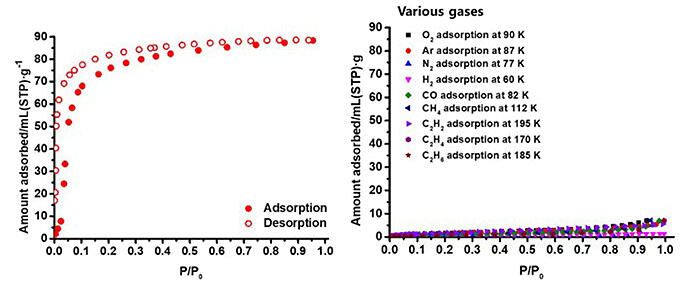
For nine gases (nitrogen, methane, carbon monoxide, oxygen, hydrogen, argon, acetylene, ethylene, and ethane), adsorption almost did not occur.
Provided by Otake
Two-component gas mixtures were created by adding equal amounts of nitrogen, methane, and ethylene to CO2, and the gas adsorbed by PCP in each gas mixture was identified. Notably, CO2 was selectively adsorbed in all the gas mixture analyses.
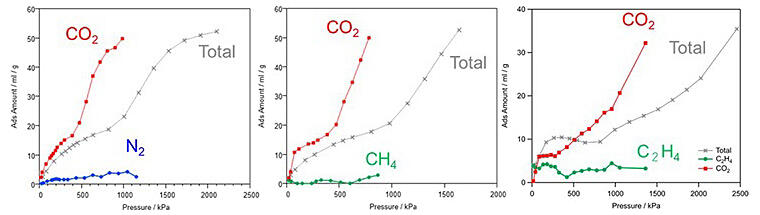
Provided by Otake
When calculating the energy loss resulting from changes caused by adsorption in the lattice structure, adsorption was a possibility, even with acetylene and ethylene. However, the gaps formed within the lattice structure after adsorption resembled a continuous pathway, where large and small gaps were interconnected. Upon calculating the energy required for gases to pass through the pathway, acetylene and ethylene were found to require more energy than CO2. Otake remarked, "Even though gases are adsorbed on the surface, only CO2 deeply penetrates the crystal's interior. The gateway appears to open exclusively for CO2 adsorption."
CO2, a greenhouse gas, can be efficiently separated and recovered by chemical adsorption, which requires high temperature. However, gas separation using porous materials is drawing attention owing to its energy efficiency. Although the separation of two-component gas mixtures has recently been the focus, Kitagawa stated, "actual factory exhaust gas and biogas contain various components other than CO2, and we are currently developing materials that can separate many similar component gases with the aim of practical application."
This study was conducted in collaboration with Tongji University in China and published in the electronic edition of the British scientific journal Nature Communications.
Journal Information
Publication: Nature Communications
Title: Soft corrugated channel with synergistic exclusive discrimination gating for CO2 recognition in gas mixture
DOI: 10.1038/s41467-023-39470-w
Original article was provided by the Science Portal and has been translated by Science Japan.




