A research group led by Professor Takashi Hibino and Associate Professor Anatoly Zinchenko of the Graduate School of Environmental Studies at Nagoya University, in collaboration with SOKEN, has announced the development of a new method for the electrolysis of lignosulfonate, a waste biomass, under mild conditions to synthesize methanol at the anode and hydrogen at the cathode. A methanol yield greater than 40% was achieved. This achievement is expected to contribute to the reduction of carbon dioxide (CO2) emissions during biomass utilization process. The results were published in the September 23, 2023 issue of the international journal Applied Catalysis B: Environmental.
Provided by Nagoya University
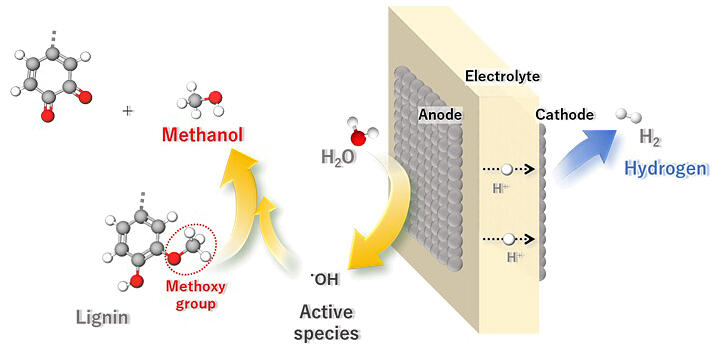
Lignin, a biomass of interest, accounts for 20−35% of the weight of wood and is separated in the form of lignosulfonates in pulp and paper mills. However, the use of lignosulfonic acid is limited, and obtaining products with high added value is challenging. Both thermal and catalytic reactions are used to convert lignosulfonic acid under high-pressure and high-temperature conditions.
In this study, the research group utilized the benefits of the electrochemical reaction and extracted methanol from lignosulfonates by anodizing the methoxy groups of these compounds at a temperature of 75℃, atmospheric pressure, and low voltage. Because the anode is exposed to acidic solutions and the potential is oxidative by nature, an electrode made of platinum (Pt), an acid- and oxidation-resistant metal, was used. Pt was sputtered onto the surface of the electrolyte membrane to increase its surface area. Commercially available carbon electrodes impregnated with nanometer-sized Pt particles were used as the cathode.
First, lignosulfonic acid (10 mg) was electrolyzed at different temperatures and anodic potentials, and the gaseous products from the anode were observed. The results showed that methanol, dimethyl ether, CO2, and oxygen were sequentially produced as the potential increased. The researchers also observed that hydrogen was produced at the cathode while methanol was extracted from the lignosulfonate at the anode. Larger amounts of lignosulfonate led to higher methanol-production rates and current efficiencies until the latter half of the reaction time. Notably, the current efficiency at any lignosulfonate content was 90% or higher and the average yield of methoxy groups to methanol was 42%.
In the developed method, methanol production via electrolytic oxidation was achieved at lower temperatures and pressures than those employed for thermal and catalytic oxidation. The oxidant is produced at the anode, the electricity can be supplied by renewable energy sources, and hydrogen is produced at the cathode as a by-product. In addition to the production of methanol, the newly developed method is expected to contribute significantly to the reduction of CO2 emissions during biomass utilization process because it can use renewable energy as a power source owing to its mild reaction conditions (75℃, atmospheric pressure) and co-produce hydrogen, which can be used as an alternative fuel.
Hibino said, "In this study, methanol and hydrogen were extracted from lignin, but aromatic compounds were left untreated at the anode. In the future, we aim to control the reaction such that this residue can be converted into phenol and other useful compounds."
Journal Information
Publication: Applied Catalysis B: Environmental
Title: Electrochemical extraction of methanol from lignin under mild conditions
DOI: 10.1016/j.apcatb.2023.123328
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




