A group of researchers of Tohoku University and the University of Tokyo has discovered that a proteolytic enzyme purified from the venom of the snake Protobothrops flavoviridis degrades the amyloid beta (Aβ) peptide, which is believed to be a cause of Alzheimer's disease. Enzymes in the human body are known to break down Aβ, but this is the first time that toxins in living organisms have been found to be effective in doing this. The finding is expected to lead to the development of new treatments for Alzheimer's disease.

Provided by the Okinawa Prefectural Institute of Health and Environment
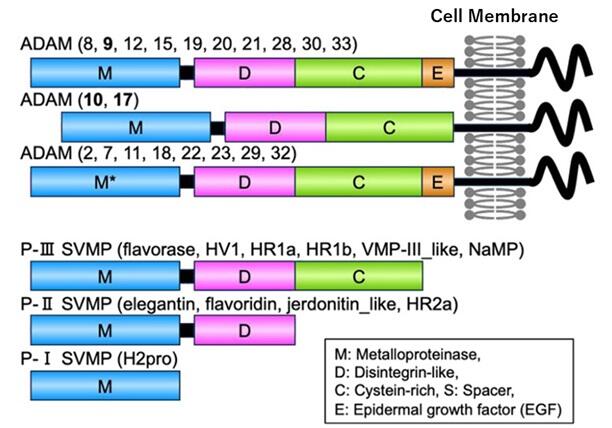
Associate Professor Eugene Futai, who studies Enzyme Chemistry, and Professor Tomohisa Ogawa (Protein Chemistry) of the Graduate School of Agricultural Science at Tohoku University have isolated and purified metalloproteinases (proteolytic enzymes) from the snake P. flavoviridis venom metalloproteinases (SVMPs) by utilizing the interaction between metal ions and proteins. The whole genome of P. flavoviridis was successfully decoded in 2018 by a group that includes Kyushu University. SVMPs are composed of many biomolecules, including 11 different metalloproteinases, and are referred to as a 'protein cocktail.' These metalloproteinases cause internal bleeding and blood clotting in humans bitten by P. flavoviridis.
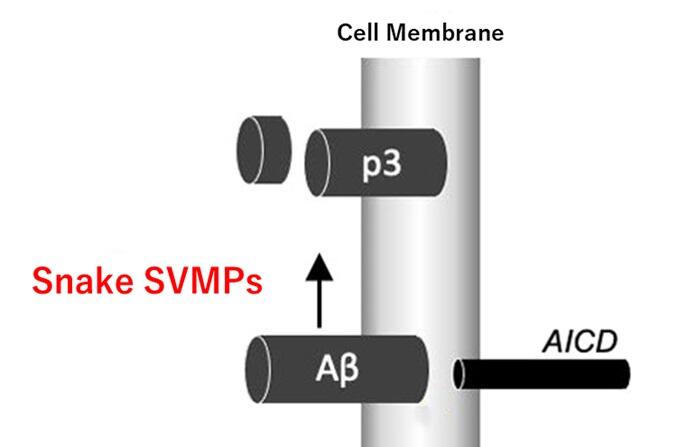
In this study, Futai and his colleagues focused on the fact that an enzyme originally found in the human body degrades Aβ. They extracted nine different types of snake venom metalloproteinases that were similar in structure to the human enzyme and are thought to have evolved from a common ancestor.
A 'cocktail' of the nine different snake venom metalloproteinases was then applied to cells secreting Aβ. The research group found that only Aβ was cleaved and broken down into peptides with harmless amino acids attached. They also observed that the production of Aβ by cultured human cells was substantially reduced.
However, when the snake venom metalloproteinase cocktail was applied at high concentrations, the toxicity was more strongly expressed in the form of cell death itself. Futai stated, "We would like to confirm in mouse experiments what concentration is most effective."
Additionally, the nine snake venom metalloproteinases were applied as a 'cocktail,' and the most effective type among them has not yet been identified.
Provided by Tohoku University
Provided by Tohoku University
SVMPs can be produced in the laboratory using the polymerase chain reaction (which amplifies DNA) or by artificial genes. If this research is pursued, it could be applied to a therapeutic agent for Alzheimer's disease.
The research was funded by a Grant-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (JSPS), and the results were published in the electronic edition of the Swiss scientific journal Toxins on August 12 and announced by Tohoku University on August 31.
Journal Information
Publication: Toxins
Title: A Metalloproteinase Cocktail from the Venom of Protobothrops flavoviridis Cleaves Amyloid Beta Peptides at the α-Cleavage Sites
DOI: 10.3390/toxins15080500
Original article was provided by the Science Portal and has been translated by Science Japan.




