A research group led by Professor Kei-ichiro Ishiguro and Assistant Professor Ryuki Shimada of the Institute of Molecular Embryology and Genetics at Kumamoto University announced that they have clarified the mechanism through which meiosis is controlled during egg formation. The research group found that in germ cells that have switched to a meiotic state during the process of egg formation from germ cells in the embryonic mouse ovary, the retinoblastoma (RB), a "tumor suppressor protein" that inhibits the activation of DNA synthesis, needs to be temporarily deactivated in the germ cells to enhance DNA synthesis at the start of meiosis. The results are expected to lead to advances in reproductive medicine and were published in the October 25 issue of the international academic journal Nature Communications.
Provided by Kumamoto University
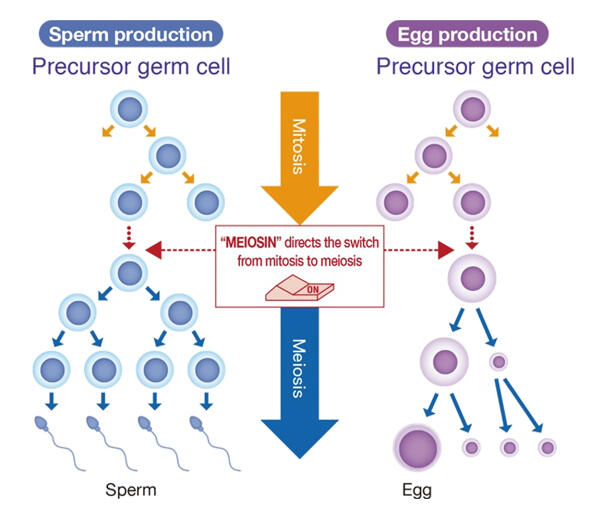
The eggs in the ovary and sperm cells in the testis are produced through meiosis, a cell division process that is different from the mitosis that occurs in other body tissues. Males produce sperm throughout their lives after puberty, but in contrast, female meiosis in the fetal ovaries begins at a very limited time during the fetal period (14−17 weeks of pregnancy) and remains dormant over the time span of the ovaries until ovulation occurs. This means that in females, only a limited number of reproductive cells that started meiosis during the fetal period can develop into eggs. However, the meiosis-initiating mechanism specific to females had been unclear.
In 2020, the research group discovered MEIOSIN, a gene that switches on meiosis. They showed that the binding of the MEIOSIN protein to another protein named STRA8 activates hundreds of genes involved in the formation of sperm cells and eggs and that the division mode of the germ cells changes from somatic cell division to meiosis. The research group used genetically modified mice in which only germ cells that had switched to meiosis would emit fluorescence. They collected only these relevant cells and analyzed them using mass spectrometry. They discovered that STRA8 binds not only to MEIOSIN but also to RB, a tumor suppressor protein.
Using genome editing, they created a female mouse in which the Stra8 gene was modified to mutate the protein's RB-binding site, thereby inhibiting STRA8 binding to RB. Examination of the ovaries showed that the resulting delay in meiotic progression caused early egg death and infertility.
To investigate this mechanism further, the research group collected a small number of cells in the ovary that were changing into the meiotic state and used the single-cell RNA-sequencing technique to examine the behavior of various genes.
They found that the genome doubling due to DNA synthesis that occurs before cells enter meiosis in normal fetal ovaries did not occur in the mouse fetal ovaries in which RB binding was inhibited. Furthermore, they also found that the activation of MEIOSIN, which occurs in normal ovaries, was not observed when RB binding was inhibited, and meiosis did not start. Additionally, with RB binding inhibited, cell death was induced in the fetal ovaries, resulting in atrophy of the ovaries.
Normally, RB suppresses genes related to DNA synthesis by binding to the transcriptional activator E2F and thereby prevents excessive cell proliferation. The binding of STRA8 to RB temporarily cancels this process. At the same time, STRA8 was speculated to bind to MEIOSIN to activate genes involved in meiosis. Previous studies have reported that ovarian tumors are caused by excessive DNA synthesis due to RB dysfunction.
Ishiguro commented, "Our discovery revealed that germ cells in the fetal ovary were controlled by a tumor suppressor protein. We expect this finding to contribute to clarification of the pathology of infertility, especially in women with oocyte formation defects. We also found that it is essential for an appropriate number of eggs to undergo meiosis at the appropriate time, suggesting that medications and foods consumed during pregnancy that affect RB may affect this function. We believe that this observation may serve as a guideline for future drug administration policies."
Journal Information
Publication: Nature Communications
Title: STRA8−RB interaction is required for timely entry of meiosis in mouse female germ cells
DOI: 10.1038/s41467-023-42259-6
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




