A joint research group led by Assistant Professor Keisei Sowa of the Graduate School of Agriculture at Kyoto University and Specially Appointed Professor Keiichi Namba of the Graduate School of Frontier Biosciences and Associate Professor Hideaki Tanaka of the Institute for Protein Research at Osaka University has successfully analyzed the structure of fructose dehydrogenase (FDH) from Gluconobacter japonicus and revealed details of its unique enzymatic reaction mechanism. Cryo-electron microscopy observation and single-particle image analysis were performed, and the structure of FDH was successfully analyzed at a resolution of 2.5 Angstroms. From this, the amino acids that play an important role in the catalytic reaction of the enzyme were identified. FDH recognizes the electrode as a substrate and initiates a unique direct electron transfer (DET)-type reaction. The findings are anticipated to lead to the development of new biosensors employing the same reaction and were published in the October 12 issue of the international journal ACS Catalysis.
Provided by Kyoto University
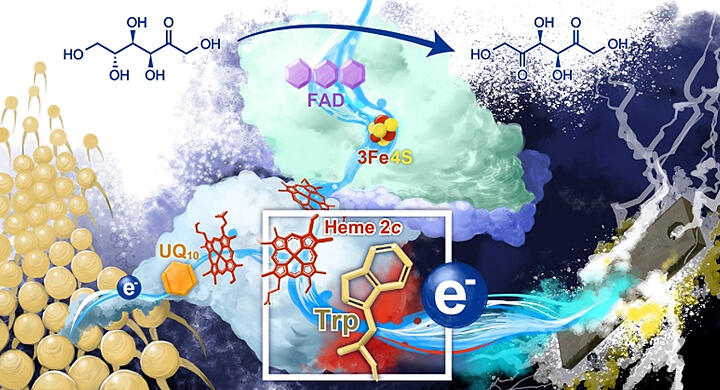
FDH, a membrane-bound protein, is a component of the respiratory chain electron transfer system of G. japonicus. As FDH achieves DET by recognizing an electrode as a substrate, it is anticipated to be developed as a new biosensor for detecting biosubstances that are highly compatible with living organisms and the environment.
In this study, the research group identified the electrode-active site of the enzyme and showed that tryptophan (an aromatic amino acid) in the enzyme promotes DET-type reactions. Furthermore, the DET-type reaction of FDH was analyzed in a mathematical model to quantitatively evaluate the electron transfer enhancement effect of tryptophan. The three-dimensional structure of FDH was clarified and the electrode-active site was identified through electrochemical evaluation. The amino acid residues responsible for the superior DET-type activity of FDH were also identified.
This is the first-ever report of the overall structure of an enzyme capable of both FDH- and DET-type reactions. The results of this study provide a basis for the development and eventual realization of new biocatalytic biodevices.
Sowa commented, "FDH was first reported as a DET-type enzyme in 1991, and I am very happy and excited that we were the first in the world to clarify its three-dimensional structure, which had been unknown for more than 30 years. Basic research on enzymes capable of achieving DET-type reactions is of great academic and social importance in building a sustainable society in the future. By utilizing the advanced catalytic functions created by nature, we will develop innovative technologies that serve to enrich humanity and the Earth and will work to apply the results of our research to society."
Journal Information
Publication: ACS Catalysis
Title: Essential Insight of Direct Electron Transfer-Type Bioelectrocatalysis by Membrane-Bound d-Fructose Dehydrogenase with Structural Bioelectrochemistry
DOI: 10.1021/acscatal.3c03769
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




