Although skeletal muscles tissue is known to show gradual age-related reduction in mass and function, the mechanism underlying these changes is not well understood. An international joint research group, including Professor Ryuichi Tatsumi, Associate Professor Takahiro Suzuki, Professor Mako Nakamura, and Assistant Professor Takashi Nakashima, all of the Faculty of Agriculture Graduate School of Bioresource and Bioenvironmental Sciences School of Agriculture, Kyushu University, and Lecturer Alaa Elgaabari of the Faculty of Veterinary Medicine at Kafrelsheikh University in Egypt discovered that hepatocyte growth factor (HGF) in muscle stem cells (satellite cells) loses its physiological activity when it is nitrated, and this phenomenon progresses and accumulates with age.
Tatsumi said, "If we can achieve the prevention of nitration, we may lead to the development of preventive and therapeutic methods not only for age-related muscle atrophy and regenerative failure but also for neurodegenerative disorders such as Alzheimer's and Parkinson's diseases." The findings were published in advance in the electronic version of the journal Aging Cell.
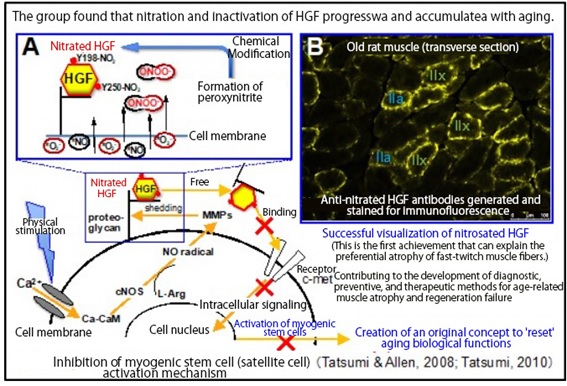
Muscle stem cells proliferate when subjected to physical stimulation. This is because HGF, which is bound and stored around muscle fibers, is released and binds to the cell membrane receptor c-met to activate muscle stem cells. This HGF-mediated activation of muscle stem cells decreases with age, leading to atrophy of the skeletal muscle (reduction in muscle mass) and an increase in connective tissue. Although many studies have reported changes in related gene expression, the fundamental mechanism that induces such change remained unclear.
Tatsumi commented, "We have been conducting research on the activation of satellite cells for approximately 25 years, but in the beginning, we could not explain why the cells were not activated. Fifteen years ago, I came up with the idea that HGF might undergone some kind of chemical modification. However, I could not fully analyze it. Following this (6 years ago), I finally speculated that HGF might be inactivated via nitration."
Nitration is a reaction in which a nitro group (−NO2) binds to the side chain of tyrosine residues. The reaction occurs when peroxynitrite, generated by the combination of NO radicals and active oxygen, binds to tyrosine residues in the protein.
The research group found that when HGF is nitrated, it loses its ability to bind to the cell membrane receptor c-met. They also demonstrated in rats that the phenomenon progresses and accumulates with age. By contrast, other cell growth factors (FGF2, IGF1, TGF-β3) that control the proliferation and differentiation of muscle stem cells were not nitrated. The research group further revealed that the tyrosine residues (Y) that underwent nitration in HGF were Y198 and Y250. These two tyrosine residues constitute the site for binding c-met.
Subsequently, the research group created monoclonal antibodies (nitrated Y198-HGF and nitrated Y250-HGF antibodies) that specifically recognize the nitrated HGF. They found that the nitration process proceeded and the nitrated HGF accumulated significantly in the fast-twitch IIa and IIx types of muscle fiber. This is the first-ever reported result that can explain the preferential atrophy of fast-twitch muscle fibers in human skeletal muscle.
The key point of the achievement comes from the creation of the monoclonal antibodies. The visualization of the nitrated Y198-/Y250-HGF, even in samples containing a variety of nitrated proteins, has greatly advanced the research. The antibodies are widely applicable to the HGFs in humans, cats, dogs, mice, rats, and other species. Applications of these are anticipated to be extended to the medical field, such as for the early diagnosis of age-related muscular atrophy in humans and pets.
Instead of the traditional method of 'reducing oxidative stress,' the research group is developing an original method that uses denitration enzymes to reset aging biological functions by 'revitalizing functions denatured by oxidative stress (rejuvenation).' To realize this concept, the group is continuing research on identifying and induction of expression of novel genes.
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




