Due to its high stored energy per unit weight (specific mass energy density), the lithium-air battery is expected to be used in lightweight devices such as drones, IoT devices, and home energy storage systems, where weight is a decisive factor. Theoretically, the lithium-air battery possesses an energy density several times greater than that of lithium-ion batteries. However, there is severe degradation within the battery, which affects the cathode, the anode, and the electrolyte. The limited number of reversible discharge-charge cycles, which is usually around a dozen, therefore poses a major challenge.
A research group, led by Professor Hirotomo Nishihara and Assistant Professor Wei Yu from the Advanced Institute for Materials Research (AIMR) at Tohoku University, collaborated with NIMS, Osaka University, Ochanomizu University, and Hokkaido University. They proposed an ideal cathode structure using a new carbon material called graphene mesosponge (GMS), and successfully achieved an ultra-high capacity and long cycle life. The results were published in Advanced Energy Materials.
©Wei Yu, Hirotomo Nishihara et al.
Provided by Tohoku University
Lithium-air batteries use a lithium metal as the anode and a porous carbon material as the cathode. During discharge, the lithium in the anode dissolves in the electrolyte as lithium ions. These lithium ions then react with oxygen molecules from the air and form lithium peroxide as a discharge product in the carbon cathode. During charging, lithium peroxide reversibly decomposes into lithium ions and oxygen molecules. Repeated discharging and charging of the lithium-air battery is accompanied by significant degradation of its major components, including the cathode, anode, and electrolyte. Researchers around the world are actively investigating solutions to prevent the degradation of each component in lithium-air batteries.
Carbon materials are currently being considered for the cathode. An ideal cathode material must provide adequate space for the deposition of lithium peroxide, act as a conductive path for the electrical flow to the insulating lithium peroxide, and have stability to prevent immediate decomposition during the discharging and charging processes. Currently, carbon is the best material that meets these conditions. However, even state-of-the-art carbon materials undergo degradation through repeated discharge and charge cycles, thereby lacking the requisite cycle life for sustained performance.
Despite extensive research into various carbon cathodes, including carbon black, activated carbon, carbon nanotubes, and graphene-based materials, none has yielded satisfactory results. To increase the battery capacity, the loading amount of lithium peroxide must be increased, which requires an increase in the amount of space (pore volume) within the cathode where lithium peroxide is deposited. However, conventional carbon materials present a trade-off between pore volume and carbon degradation resistance, making it challenging to achieve both goals simultaneously.
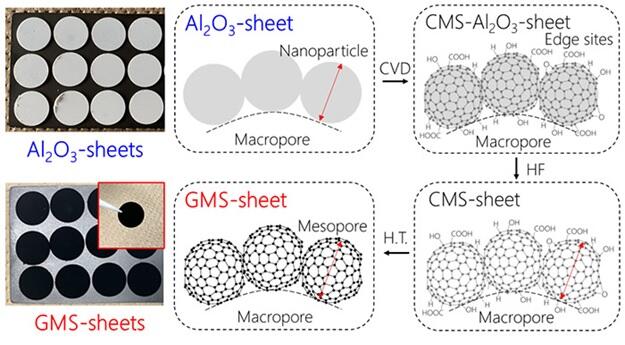
In this study, the research group focused on graphene, the basic unit of all carbon materials, to address the long-standing challenge of balancing high capacity and extended cycle life in the carbon cathode of lithium-air batteries. Utilizing the unique chemical characteristics of graphene, they proposed an ideal cathode structure. Specifically, the cathode structure was designed to have a large pore volume, thereby enhancing its capacity significantly. Additionally, efforts were made to eliminate graphene layering, effectively reducing the weight of the carbon cathode and battery. Moreover, careful consideration was given to eliminating degrading sites (i.e. edges), to achieve an extended cycle life. A self-standing GMS-sheet, a new carbon material, makes such ideal structure possible.
First, alumina nanoparticles, employed as a template material, are pelletized into self-standing Al2O3-sheets. In the following process, approximately one layer of graphene is coated on the surface of Al2O3 nanoparticles during chemical vapor deposition (CVD). Subsequently, the Al2O3 nanoparticles are removed by chemical etching using hydrofluoric acid or other chemicals to obtain porous carbon in pellet form. During annealing of this sample at 1800 ℃, a zipping reaction removes the edge sites of the graphene that cause battery degradation, resulting in a self-standing GMS-sheet with extremely high degradation resistance.
Optimizing the structure of GMS-sheet contributes to a high performance. Two GMS-sheet samples after optimization exhibit particularly high performance, ultra-high mass and areal capacities, compared to that of carbon cathodes reported in previous literature. It is also confirmed that GMS-sheet not only exhibits a larger capacity than conventional carbon materials but also demonstrates a superior cycle life than traditional carbon materials. Actually, GMS is the only carbon material capable of forming a three-dimensional pore structure with non-stacked graphene, thereby maximizing pore volume while simultaneously eliminating edge sites that cause degradation. Therefore, this material represents an ideal form of carbon cathode for lithium-air batteries.
In this study, the research group has achieved the most favorable structure for the carbon cathode. However, when employing an the practically-high current density and limited capacity necessary for practical applications, it can only undergo discharging and charging approximately 21 times. This is possibly due to the significant degradation of the anode and the electrolyte. Future advances are anticipated to extend the cycle life of lithium-air batteries with GMS cathodes, provided that improvements in both the anode and electrolyte are actively pursue.
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




