A research team at Keio University and Kyoto University has discovered a new therapeutic approach focusing on metabolic changes in myocardial infarction. The team was led by Assistant Professor Genki Ichihara of the School of Medicine (at the time of research), Associate Professor Motoaki Sano of the School of Medicine, Assistant Professor Yoshinori Katsumata of the Institute for Integrated Sports Medicine at the School of Medicine at Keio University, and Program-Specific Associate Professor Yuki Sugiura of the Graduate School of Medicine at Kyoto University. Using a mouse model of the disease and techniques for monitoring metabolic molecules, the research team observed the stepwise process of ischemia-reperfusion injury in the myocardium in detail.
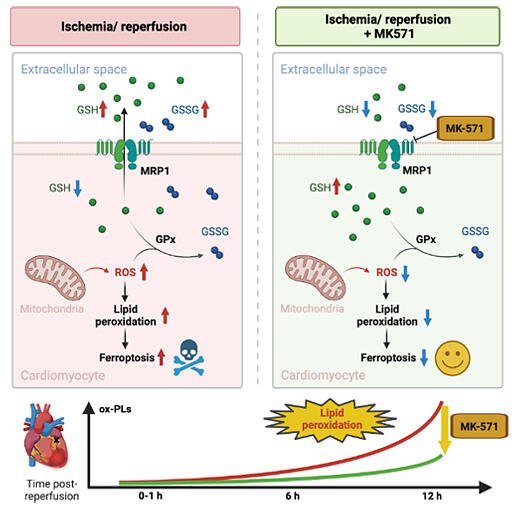
They found that ischemia-reperfusion injury was caused by the extracellular release of glutathione (an important reducing factor in myocardial protection) via a transporter protein known as multidrug resistance protein 1 (MRP1). Interventions in pathways that enhance the removal of reactive oxygen species can also reduce myocardial damage after ischemia-reperfusion injury. The results are expected to lead to the development of treatments that support recovery from myocardial infarction. The results of this study were published in the October 11 issue of Circulation Research.
Provided by Keio University
Myocardial infarction (an ischemic heart disease that causes tissue necrosis due to insufficient blood supply) is the leading cause of death worldwide. However, recent improvements in treatment techniques have significantly increased the patient survival rate. However, many patients are left with heart failure after rescue due to impaired heart pumping function caused by the ischemia-reperfusion injury that occurs with myocardial infarction, and there is no established treatment for this problem.
Ischemia-reperfusion injury occurs when blood flow is restored to the ischemic myocardium, and the rapid reoxygenation produces large amounts of reactive oxygen species that alter various intracellular proteins. In this study, the research team reproduced the ischemia-reperfusion injury in rat hearts and continuously analyzed the metabolite fluctuations that occur during the process in the living state. A tubular semipermeable membrane was implanted in the heart wall, and a technique (microdialysis) was used to continuously collect and measure metabolites in the myocardial interstitium that permeates this membrane. Traditionally, target molecules have been sought by collecting cardiomyocytes after injury and analyzing the metabolites. However, it was not possible to follow the rapidly changing metabolic state of the myocardium over time.
As a result of the new technique used in this present study, metabolites in the myocardial interstitium were successfully recovered continuously in the same living individual for each of the following three time phases: before ischemia, during ischemia, and after reperfusion. The polypeptide glutathione, a key factor in myocardial protection and a potent reductant, is released from the cell during ischemia-reperfusion and is unable to remove intracellular reactive oxygen species. The depletion of intracellular glutathione increases reactive oxygen species and causes ferroptosis (an iron-dependent cell death process) due to lipid peroxidation and excessive oxidation of lipids in the cell membrane. Additionally, this extracellular release of glutathione was found to be primarily mediated by MRP1, a specialized transporter.
When MRP1 function was inhibited by drugs, glutathione was retained in the cells, and it not only scavenged reactive oxygen species but also reduced oxidized lipids, thereby supporting cardiomyocyte survival. Furthermore, this treatment targeting ferroptosis was found to have some efficacy even when initiated several hours after ischemia-reperfusion injury.
Targeting the increase in oxidized lipids observed after 6 hours of myocardial infarction is expected to lead to effective treatment by creating a time advantage before the administration of therapeutic agents.
Katsumata stated, "The new technology used in this study — a method for monitoring metabolic molecules using dialysis membranes − enables detailed observation of changes in metabolites during the stepwise process of ischemia-reperfusion injury in the heart of living mice. The discovery that glutathione, a key molecule in the defense mechanism against oxidative stress, leaks out of cells during ischemia and reperfusion was very exciting. We hope that this discovery will lead to a new treatment for myocardial infarction."
Journal Information
Publication: Circulation Research
Title: MRP1-Dependent Extracellular Release of Glutathione Induces Cardiomyocyte Ferroptosis After Ischemia-Reperfusion
DOI: 10.1161/CIRCRESAHA.123.323517
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




