A research group led by Assistant Principal Researcher Yurina Sekine, Researcher Takuya Nankawa, Assistant Principal Researcher Kosuke Hiroi, Researcher Tsuyoshi Sugita, and Postdoctoral Researcher Yuki Shibayama at the Materials Sciences Research Center, Japan Atomic Energy Agency (JAEA), in collaboration with Toyohashi University of Technology, Tokyo Metropolitan Industrial Technology Research Institute, and Meiji University, has discovered that a high-strength porous gel material can be produced by mixing cellulose nanofibers made from unraveled pieces of wood with an extremely low concentration of sodium hydroxide, freezing the mixture, and dissolving it by adding citric acid.
Sekine stated, "We believe that the development of materials through chemical reactions using ice crystal space can be used in various fields. We aim to expand this development to different areas, including resource recycling." The results were published in the journal Carbohydrate Polymers.
Provided by JAEA
Cellulose, a component of wood, has long been used in chemical and physical processing for fabricating chemical products and clothing. However, its use requires a significant amount of energy to disrupt the microscopic hierarchical structure in which cellulose molecules are arranged.
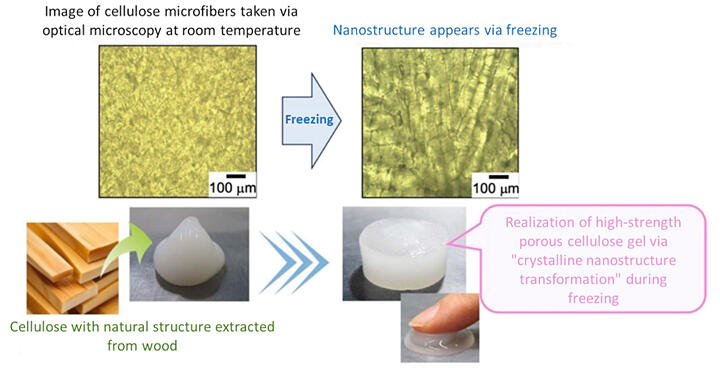
The research group focused on utilizing the nanospace enclosed between ice crystals formed during the freezing of water. When the cellulose solution freezes, the cellulose molecules are not incorporated into the ice crystals, resulting in the formation of a dense, nanometer-sized agglomerated layer of cellulose between the ice crystals. In this agglomerated layer, the molecules are in close proximity to each other, resulting in a unique molecular arrangement compared to the typical arrangement.
Through this study it was found that sodium hydroxide acts in the nanospaces created between ice crystals during the freezing of aqueous solutions, causing a phase transition in the crystal structure from the naturally occurring cellulose I, in which the cellulose molecules are arranged in parallel, to cellulose II, in which the cellulose molecules are arranged in opposite directions and are connected by hydrogen bonds.
Then, by adding citric acid to the aqueous solution in a dissolution phase, carboxyl groups are introduced into the cellulose and hydrogen bonds are formed between the hydroxyl groups to form a reticulated crystal structure. This resulted in the formation of a strong gel. Cellulose porous gel materials are highly permeable with more than 95% porosity, allowing water and other substances to move in and out of the interstices.
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




