Iron is an attractive catalytic material because of its abundance in nature, low cost and low toxicity. However, conventional iron catalysts are significantly less active in hydrogenation reactions under mild liquid-phase conditions of less than 200 ℃. In addition, the active species, iron nanoparticles, are easily oxidized by even a small amount of oxygen remaining in the reaction system, causing them to lose their activity. Given this low activity and instability, iron catalysts can only be used in very limited situations, such as gas-phase reactions which require high temperatures.
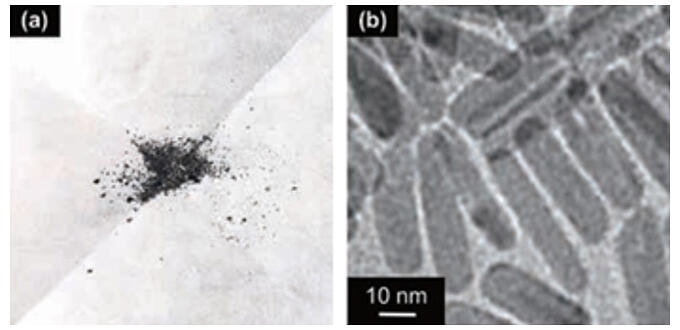
A research group led by Associate Professor Takato Mitsudome of the Graduate School of Engineering Science at Osaka University has synthesized iron phosphide nanocrystals, which are composed of iron and phosphorus, with a size of less than 30 nm. They were found to be the most active solid iron-based catalysts developed to date for the hydrogenation of industrially important nitriles under liquid-phase reactions below 200 ℃.
The developed nanocrystals are stable in air, which makes them easy to handle, and they can be easily applied to improve catalysts. Taking advantage of this feature, the catalytic activity of the obtained nanocrystals was dramatically improved when they were combined with titanium dioxide. This enabled the selective synthesis of primary amines from a variety of nitriles. Primary amines are important as pharmaceutical intermediates and as raw materials for polymers. Furthermore, the research group confirmed that the catalyst can be easily separated from the reaction solution after the reaction and can be reused repeatedly while maintaining high activity.
This is the first iron catalyst that exhibits air-stability, high activity and durability in the liquid phase hydrogenation reactions. The results also suggest the possibility of sustainable next-generation chemical reaction processes that do not use rare metals, whose applications are limited by concerns regarding their scarcity and toxicity. The developed iron phosphide nanocrystals also exhibited a high hydrogenation capacity. As a result, they are expected to be applied in biomass conversion and polymer degradation reactions with an eye toward carbon neutrality.