Despite increasing survival rates for many cancer types, pancreatic cancer remains the most difficult cancer to treat, with a 5-y survival rate of only 6%, with treatment often being too late to save patients when the disease is detected. A research group led by Dr. Tomonori Masuda, Associate Professor Akihisa Fukuda and Professor Hiroshi Seno, all from the Department of Gastroenterology and Hepatology at the Graduate School of Medicine, together with Professor Emeritus Makoto Noda of the Department of Molecular Oncology at the Graduate School of Medicine at Kyoto University, have determined that the expression level of the membrane protein RECK plays a major role in pancreatic cancer by decreasing the onset and metastasis of the disease. Noda commented, "There are only a few cases where the RECK gene is mutated in cancer. The gene is only inactivated, not broken; thus, the administration of the activating drug may lead to new treatments for pancreatic cancer." The results were published online in the Journal of Clinical Investigation.
Provided by Kyoto University
RECK (Reversion-inducing Cysteine-rich protein with Kazal motifs) is a membrane protein that was discovered while searching for a gene that restores normal mouse fibroblasts from the malignant state caused by the oncogene Kras. RECK, which has multiple functional domains, suppresses protein degradation on the cell surface and assists in signal transmission. Although mutation of this gene is minor in many cancer cells, gene expression levels are known to be lower than that in normal healthy cells.
The research group created KPC (Kras, p53, and Cre) mice, which had multiple genetic mutations that lead to pancreatic cancer. In the KPC mice, RECK was expressed in precancerous lesions, but its expression was significantly reduced or disappeared in the cells of pancreatic cancer tissue, which was analogous to that of humans.
A Kras gene mutation found in almost all human pancreatic cancer patients was introduced in KC (Kras, Cre) mice and only 10% of the rodents developed pancreatic cancer. In addition to the Kras mutations, specific knockout of the RECK gene in the pancreas resulted in a high incidence of pancreatic cancer development and liver metastasis.
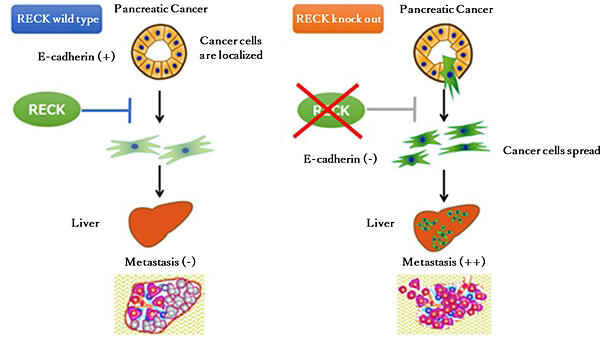
In the pancreatic cancer cells that developed in RECK-knockout mice, the expression of E-cadherin (necessary for cell-cell adhesion) was markedly reduced, and the cells were histologically changed into a mesenchymal type that was prone to invasion. Furthermore, pancreatic cancer developed in all the KC mice with p53 heterozygosity, but liver metastasis did not occur. However, liver metastasis occurred at a high rate when RECK was knocked out. In other words, RECK was shown to suppress the onset and metastasis of pancreatic cancer.
Using the control KPC mice that developed pancreatic cancer, the researchers then performed lineage tracing of pancreatic epithelial-derived cells. As a result, in the pancreatic cancer tissues of these mice, no pancreatic epithelial cells were observed in the stroma. However, when RECK was knocked out, many stromal cells derived from the pancreatic epithelium and negative for E-cadherin were detected. In other words, it was revealed that in the body of the mouse, RECK knockout changed the properties of pancreatic cancer cells from the epithelial to the mesenchymal type.
Additionally, when the RECK gene was re-introduced into the RECK-knockout pancreatic cancer cells using retroviruses, the invasive ability of the cells was reduced. The detailed analysis using a liver metastasis model revealed that in RECK-knockout pancreatic cancer cells, the expression of E-cadherin was reduced, and the properties of the cells changed from the epithelial to the mesenchymal type. Knocking out RECK increased the expression of matrix metalloproteinases (MMP2/MMP3; enzymes that cleave cell surface proteins) and decreased the expression of E-cadherin.
Analysis of pancreatic cancer tissue from patients also confirmed that human pancreatic cancers with low RECK expression show weak E-cadherin expression, frequent distant metastasis, low differentiation, and poor prognosis. Additionally, no RECK protein was expressed in any of the nine types of human pancreatic cancer cell lines analyzed.
These results indicate that RECK increases the cellular expression of E-cadherin and suppresses the onset, epithelial-mesenchymal transition, and metastasis of pancreatic cancer. Moreover, it implies that the restoration of RECK expression suppresses pancreatic cancer metastasis.
Fukuda explained, "Since we have already found a low-molecular-weight compound that increases the expression of RECK, we will use this compound as the base to develop drugs that suppress the invasion, metastasis, and proliferation of pancreatic cancer cells."
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




